 |
by Evan Ackerman on (#7427K)
|
 IEEE Spectrum
IEEE Spectrum
| Link | https://spectrum.ieee.org/ |
| Feed | http://feeds.feedburner.com/IeeeSpectrum |
| Updated | 2026-03-07 04:15 |
 |
by Tom Clynes on (#7424S)
|
 |
by MathWorks on (#741ZS)
|
 |
by Kathy Pretz on (#741GR)
|
 |
by Willie D. Jones on (#740GM)
|
 |
by Margo Anderson on (#740DV)
|
 |
by Alexander C. Kaufman on (#73ZVP)
|
 |
by Elizabeth Fuscaldo on (#73ZQ6)
|
 |
by MathWorks on (#73Z88)
|
 |
by Benjamin Skuse on (#73YSK)
|
 |
by Peter Fairley on (#73YH1)
|
 |
by Chi Chen on (#73YDT)
|
 |
by Missy Cummings on (#73YDV)
|
 |
by Mary Ellen Randall on (#73Y1C)
|
 |
by Eliza Strickland on (#73XQ8)
|
 |
by Willie D. Jones on (#73XDN)
|
 |
by Allison Marsh on (#73X95)
|
 |
by Evan Ackerman on (#73WQ1)
|
 |
by Master Bond on (#73WEC)
|
 |
by Willie D. Jones on (#73VWZ)
|
 |
by Vanessa Bates Ramirez on (#73VT6)
|
 |
by Wiley on (#73VPT)
|
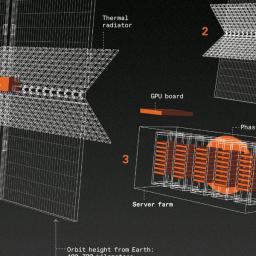 |
by Glenn Zorpette on (#73VPV)
|
 |
by Boston Micro Fabrication on (#73VHR)
|
 |
by Brian Jenney on (#73TZ8)
|
 |
by Benjamin Skuse on (#73TVP)
|
 |
by Rohan S. Puranik on (#73TVQ)
|
 |
by Liz Wegerer on (#73T3H)
|
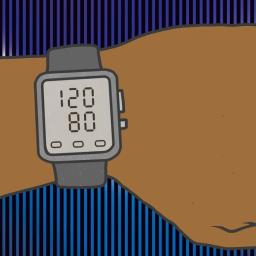 |
by Samuel K. Moore on (#73SXS)
|
 |
by Dina Genkina on (#73RZ8)
|
by Johns Hopkins University on (#73RT1)
 |
by Waydell D. Carvalho on (#73RT2)
|
 |
by Paul Jones on (#73RA0)
|
 |
by Drew Robb on (#73QT8)
|
 |
by Kathy Pretz on (#73QC1)
|
 |
by Evan Ackerman on (#73QC2)
|
 |
by Vanessa Bates Ramirez on (#73PDV)
|
 |
by Angelique Parashis on (#73NPS)
|
 |
by Reza Ghodssi on (#73NHD)
|
 |
by Willie D. Jones on (#73MVW)
|
 |
by Arjun Sharma on (#73MGF)
|
 |
by Gwendolyn Rak on (#73KV6)
|
 |
by Evan Gough on (#73K52)
|
 |
by Willie D. Jones on (#73JRB)
|
 |
by Robert Schneider on (#73J8S)
|
 |
by Evan Ackerman on (#73J8T)
|
 |
by Rahul Rao on (#73HA3)
|
 |
by Catherine Arnold on (#73H6C)
|
 |
by Joanna Goodrich on (#73GHG)
|