New study hints that we’re closing in on metallic hydrogen
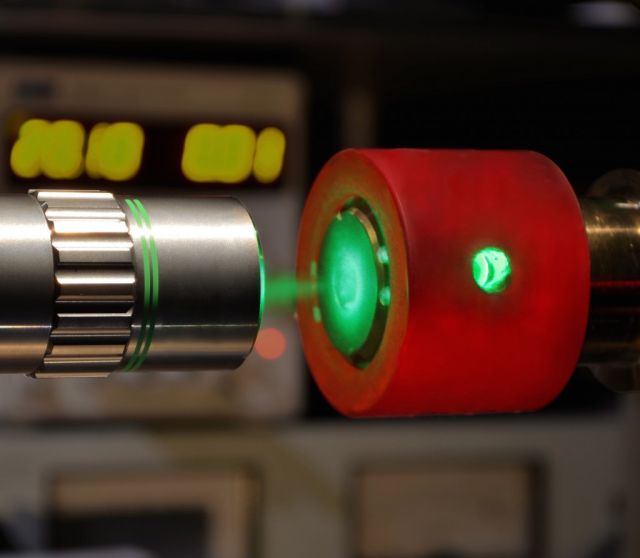
Raman spectroscopy and a diamond anvil were used to identify and characterize a new phase of hydrogen. (credit: Philip Dalladay-Simpson and Eugene Gregoryanz)
We tend to think of the properties of the chemical elements as immutable-a metal is a metal, and a gas is a gas. But all those properties are what we experience on Earth. The Universe, on the other hand, is filled with extremes of temperature and pressure that cause elements to defy our expectations. Pluto is covered in nitrogen ice, while some exoplanets are likely to experience rains of liquid metal.
Close to the cores of gas giants, elements are squeezed by unimaginable pressures, capable of rearranging electron orbitals and playing havoc with the chemical bonding we see on Earth. Here, theorists have predicted, the electrons of hydrogen could be set free, converting the gas into a solid or liquid metal. But while researchers first predicted the existence of metallic hydrogen 80 years ago, the element has stubbornly refused to appear, even after we've raised pressures well above where it was expected to appear.
Now, three Edinburgh-based researchers report placing hydrogen under the highest pressures yet achieved. Scans of the resulting material suggest that the chemical bonds that normally link hydrogen into a molecule are starting to break down, heralding the possible appearance of a metallic form.
Read 9 remaining paragraphs | Comments