Using Neurostimulation to Treat ADHD Isn't as Scary as It Sounds
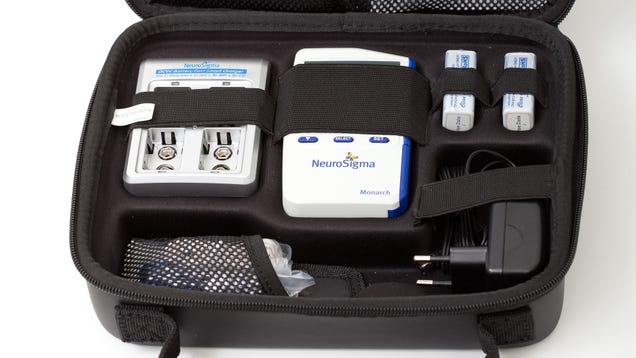
Last week, the Food and Drug Administration cleared the Monarch external Trigeminal Nerve Stimulation (eTNS) system, a medical device that provides mild brain stimulation to treatment attention deficit hyperactivity disorder in children ages 7 to 12.