Turning Bricks Into Supercapacitors
As solar panels and wind turbines multiply, the big problem is how with how to store all the excess electricity produced when the sun is up or the wind blowing so it can be used at other times. Potential solutions have been suggested in many forms, including massive battery banks, fast-spinning flywheels, and underground vaults of air. Now a team of researchers say a classic construction material-the red fired brick-could be a contender in the quest for energy storage.
The common brick is porous like a sponge, and it's red color comes from pigmentation that is rich in iron oxide. Both features provide ideal conditions for growing and hosting conductive polymers, Julio D'Arcy and colleagues have found. The team at Washington University in St. Louis transformed basic blocks into supercapacitors that can illuminate a light-emitting diode.
Supercapacitors are of interest because, unlike batteries, they can deliver blindingly fast bursts of power and they recharge quickly. The downside is that, kilogram for kilogram, they store relatively little energy compared to batteries. In an electric vehicle, a supercapacitor supports acceleration, but the lithium-ion module is what provides power for hundreds of miles. Yet many scientists and technology developers are hoping supercapacitors can replace conventional batteries in many applications, owing to the steep environmental toll of mining and disposing of metals.
The building brick proof-of-concept project presents new possibilities for the world's many brick walls and structures, said D'Arcy, an assistant professor of chemistry at Washington University. Rooftop solar panels connected by wires could charge the bricks, which in turn could provide in-house backup power for emergency lighting or other applications.
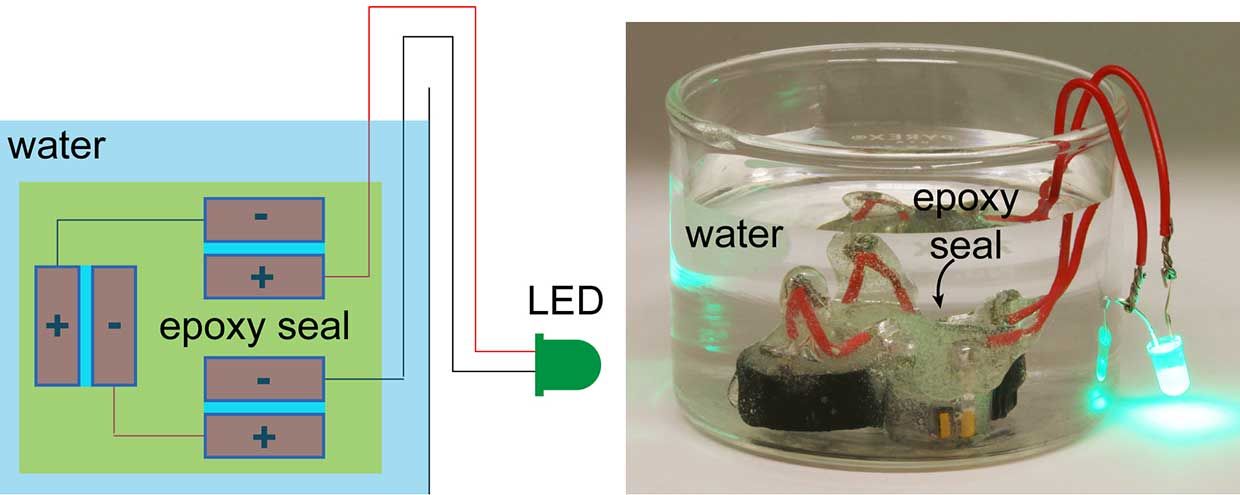 Image: D'Arcy Laboratory/Washington University in St. Louis A brick supercapacitor coated with a simple five-minute epoxy is immersed in water, demonstrating the device's impermeability.
Image: D'Arcy Laboratory/Washington University in St. Louis A brick supercapacitor coated with a simple five-minute epoxy is immersed in water, demonstrating the device's impermeability. If we're successful [in scaling up], you'd no longer need batteries in your house," he said by phone. The brick itself would be the battery."
The novel device, described in Nature Communications on Tuesday, is a far cry from the megawatt-scale storage projects underway in places like California's desert and China's countryside. But D'Arcy said the paper shows, for the first time, that bricks can store electrical energy. It offers food for thought" in a sector that's searching for ideas, he noted.
Researchers began by buying armfuls of 65-cent red bricks at a big-box hardware store. At the lab, they studied the material's microstructure and filled the bricks' many pores with vapors. Next, bricks went into an oven heated to 160 Celsius. The iron oxide triggered a chemical reaction, coating the bricks' cavities with thin layers of PEDOT, the polymer known as poly(3,4- ethylenedioxythiophene).
Bricks emerged from the oven with a blackish-blue hue-and the ability to conduct electricity.
D'Arcy's team then attached copper leads to two coated bricks. To stop the blocks from shorting out while stacked together, the researchers separated the blocks with a thin plastic sheet of polypropylene. A sulfuric-acid based solution was used as a liquid electrolyte, and the bricks were connected via the copper leads to a AAA battery for about one minute. Once charged, the bricks could power a white LED for 11 minutes.
If applied to 50 bricks, the supercapacitor could power 3 watts' worth of lights for about 50 minutes, D'Arcy said. The current set-up can be recharged 10,000 times and still retain about 90 percent of its original capacitance. Researchers are developing the polymer's chemistry further in an effort to reach 100,000 recharges.
However, the St. Louis researchers are not alone in the quest to use everyday (if unusual) materials to make supercapacitors.
In Scotland, a team at the University of Glasgow has developed a flexible device that can be fully charged with human sweat. Researchers applied a thin layer of PEDOT to a piece of polyester cellulose cloth that absorbs the wearer's perspiration, creating an electrochemical reaction and generating electricity. The idea is that these coated cloths could power wearable electronics, using a tiny amount of sweat to keep running.
The Indian Institute of Technology-Hyderabad is exploring the use of corn husks in high-voltage supercapacitors. India's corn producing states generate substantial amounts of husk waste, which researchers say can be converted into activated carbon electrodes. The biomass offers a potentially cheaper and simpler alternative to electrodes derived from polymers and similar materials, according to a recent study in Journal of Power Sources.
However, to really make inroads into the dominance of batteries, where a chemical reaction drives creation of a voltage, supercapacitors will need to significantly increase their energy density. D'Arcy said his electrically charged bricks are two orders of magnitude away" from lithium-ion batteries, in terms of the amount of energy they can store.
That's another thing we're trying to do-make our polymer store more energy," he said. A lot of groups are trying to do this," he added, but they didn't do it in bricks."