Einstein’s Fridge: Who knew the history of thermodynamics was so much like high school?
 Almost 50 years ago I had the misfortune to take two statistics classes at the same time. One was a required introduction to statistics and the other was econometrics. Don't ask why I took them both - I don't remember. But I do remember one day in the Intro to Statistics class when another student asked about this distribution plot (below).
Almost 50 years ago I had the misfortune to take two statistics classes at the same time. One was a required introduction to statistics and the other was econometrics. Don't ask why I took them both - I don't remember. But I do remember one day in the Intro to Statistics class when another student asked about this distribution plot (below).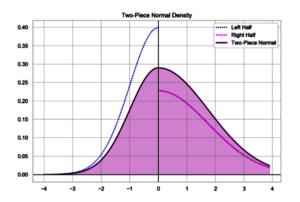
What was it? What did it indicate? What could it be used for?" they asked.
It's nothing," said the TA. It's useless."
But I had seen that shape before, in econometrics, where they called it a split normal distribution. that was said to be good for displaying time-series data.
So not useless at all.
The split-normal distribution was first drawn in 1897 and has been rediscovered several times since, most recently in 2016 by people who - honest to God - think they invented the thing.
Which brings us to an important book on the history of thermodynamics by Paul Sen. If you are into the history of science, read this book.
When I was a kid I loved reading books about science, the history of science, biographies of scientists - anything I could get my hands on about science. In all of this reading about science, though, I never read a non-textbook specifically about thermodynamics - until now.
Einstein's Fridge by Paul Sen is the thermodynamics book I never knew I needed to read. It's an exciting story of how the three laws of thermodynamics haltingly came to be and what they mean for our modern life, which comes down to pretty much everything. Thermodynamics turns out to rule both the beginning and the end of the universe. Who knew?
Author Sen comes to book writing from British television where he has made films about science and technology for more than 30 years, so the fact that he wrote this complex story as the equivalent of a really good BBC Connections episode shouldn't surprise.
To be clear, the author is my friend. He directed my documentaries Triumph of the Nerds, Connected, Plane Crazy,and of course the derivative Steve Jobs - The Lost Interview.But in Plane Crazy I threatened on camera to beat the shit out of Sen, so if I didn't legitimately like his book I wouldn't be writing this column at all.
The big players on Sen's thermodynamics stage start with Nicolas Sadi Carnot, the Frenchman who figured out that steam engines worked by moving heat around. Carnot then dropped the ball by dying of cholera at age 36. Then there was Rudolf Clausius, a German physicist who came up with the First Law of Thermodynamics, which states the equivalence of heat and work. Whenever work is done by heat then an equivalent amount of heat is consumed.
Clausius also developed the concept of entropy (the state of disorder or randomness in a system) leading to the Second Law of Thermodynamics, which states that there is a natural tendency for any system to degenerate into a more disordered state, maximizing entropy.
My son Fallon, 15, has been using entropy lately to explain why his room is so messy.
The Second Law of Thermodynamics was stated about the same time by Clausius and by the Englishman William Thomson, who was later dubbed Lord Kelvin.
The Third Law of Thermodynamics extends the concept of the Second Law, stating the entropy of a system approaches a constant value as the temperature approaches absolute zero. Another way of explaining this is that atoms stop moving at absolute zero, which pretty much describes the end of the world, eh?
This Third Law, which was stated by chemist Walter Nernst in 1906, is a sort of outlier in Sen's thermodynamics tale, both because it was coined by a chemist rather than a physicist, but also because it came after a three-decade fight-to-the-death between physicists arguing about whether atoms existed or not.
The Third Law might have come from Austrian physicist Ludwig Boltzmann. Boltzmann did the math, but that math was dependent on the existence of atoms and a lot of the cool physics kids - specifically the Germans Ernst Mach and Max Planck - did not believe in atoms. Boltzmann had developed a statistical approach that allowed him to sort of sneak up on the Third Law, but Mach and Planck didn't buy it. Like high school bullies, they taunted Boltzmann at conferences, ultimately leading to the Austrian's suicide in 1906.
I am not making this up.
Now the irony really begins to boil, because the math that Boltzmann couldn't do had already been done by Yale physics professor J. Willard Gibbs. Gibbs was able to prove the Third Law without mentioning atoms at all, but hardly anyone in Europe had even heard of J. Willard Gibbs.
The great Scottish physicist James Maxwell knew about Gibbs, writing the American was better than all the Germans," but then Maxwell died in 1879 and Gibbs lost his champion, decades before finishing his seminal work.
Gibbs was a Yale professor, but his position was unpaid and he worked in a department that had no laboratory budget. So Gibbs - who was independently wealthy - built a lab in the attic of his New Haven home. Even his scientific papers were published obscurely by the Connecticut Academy of Arts and Sciences - a publisher who literally did not understand what Gibbs was writing about.
Gibbs published his papers where he did because it was the closest thing in New Haven to a scientific journal. And the Connecticut Academy published his papers because Gibbs submitted them. They not only had no idea what he was saying, the Academy had to raise extra funds to typeset the papers (this was all math, remember) which ran as long as 471 pages!
They saw publishing Gibbs's work as a patriotic duty - and they were correct.
Einstein later described Gibbs in a wonderful bit of European chauvinism as the greatest mind in American history."
So Gibbs was an unheralded (and unread) genius who could have saved Boltzmann from the bullying of Mach and Planck, which brings us back to the split normal distribution.
Because while Mach and Planck and even Einstein were uncertain about whether atoms actually existed, their existence was, by the time of Boltzmann's suicide, already the basis of the global chemical, munitions, and pharmaceutical industries.
Like those econometricians who used split normal distributions every day, thousands of chemists IN GERMANY were relying on the existence of atoms to make their living. Which in part explains why it was a chemist who published in 1906 the Third Law of Thermodynamics.
How could the German physicists have not known this?
Einstein eventually proved the existence of atoms based on Planck's work on quanta which led to the latter's 1919 Nobel Prize - a prize that ironically could only have been won if atoms were real.
Boltzmann made possible Planck's Nobel, yet Boltzmann was still dead.
It's all in Sen's book and well worth reading.
The post Einstein's Fridge: Who knew the history of thermodynamics was so much like high school? first appeared on I, Cringely.

Digital Branding
Web DesignMarketing