REZZAYO approved by FDA amid rapid Candida auris spread
by News Desk from Outbreak News Today on (#6A33E)
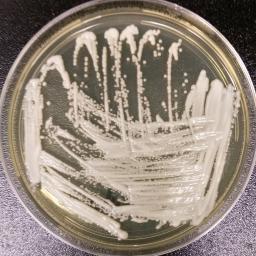
NewsDesk @bactiman63 On Monday, the CDC reported an increasing threat of the emerging fungus Candida auris. Today, the U.S. FDA today approved Cidara Therapeutics and Melinta Therapeutics' REZZAYOTM (rezafungin for injection), a novel once-weekly echinocandin, for the treatment of candidemia and invasive candidiasis in adults 18 years of age or older who have limited or no [...]
The post REZZAYO approved by FDA amid rapid Candida auris spread appeared first on Outbreak News Today.