This biotech CEO decided to take her own (fertility) medicine
To be a great company founder, they say you should use your own product. Eat your own dog food. But what if you are running a biotech company developing an experimental fertility treatment? You might be excused.
Not Dina Radenkovic, CEO of Gameto, a New York startup engineering stem cells to craft a lightweight" version of IVF-one it thinks could appeal to professional women without time to spare. Last December, the Serbian-born doctor, who is 28, found herself at home looking at a needle loaded with hormones. She pushed it under her skin and pressed the plunger.
Radenkovic wasn't trying to get pregnant. Instead, she'd signed up for her own company's medical study of how to mature" human eggs in a lab dish instead of inside their bodies. In a regular IVF process, women inject powerful hormones twice a day for two weeks in order to hyperstimulate their ovaries and generate a crop of ripe eggs, not just the usual one. And it's an ordeal: shots hurt, there can be side effects and mood swings, and the drugs cost around $6,000.
Gameto's process, described last week in the journal Human Reproduction, needs many fewer shots and uses lab-made ovary cells to complete the maturation process in a petri dish. The study involved 67 women, including Radenkovic, and tracked the development of several hundred eggs. Some were fertilized to make embryos, but none were transferred to make a pregnancy.Radenkovic says she joined the study to see how it meshed with her professional schedule.
I'm not going to recommend it to anybody as a spa day; it's still a medical procedure," she says, But I felt that it was still something I was able to integrate into a busy lifestyle of a startup CEO who is working pretty hard."
I'd spoken to Radenkovic a year ago, when she'd told me how she had hired an advisory board and raised funds-important milestones for a young company and a new executive. Like me, though, some of her contacts weren't aware she'd joined the experiment and hadn't noticed the Instagram selfie she posted from a medical procedure room, smiling and dressed in a hospital gown.
It's like Elon getting into a rocket. That means she's a badass, in my opinion," says Joe Betts-LaCroix, an angel investor in the company who leads a separate biotech, Retro Biosciences.
Unfair agingGameto is among a group of startups that see female fertility as an aging problem. While life expectancy is getting longer-it has been slowly rising for a hundred years-that's not true of women's reproductive life spans. Nearly all women run out of eggs during their 40s, and menopause follows. Radenkovic believes such accelerated ovarian aging" is unfair, causing difficult choices between children, careers, and relationships. After a divorce, she notes, a man can start a new family. A woman might not be able to.
This is a big problem and we are going to fight it with science," Radenkovic declared in a Twitter thread early last year when she announced her position as CEO of the startup, which had been operating in stealth mode since being incorporated two years earlier by a fertility entrepreneur, Martin Varsavsky, who is still its chairman.
One strategy for fighting ovarian aging is to freeze eggs while you're young to use them later in life. That can add a decade to a person's reproductive window. But when Radenkovic considered taking that step a few years ago, she was dissuaded by the time commitment. She was then a new arrival in New York City and juggling three jobs. She decided that people inclined to plan ahead, with $10,000 to spend (that's about what it costs), are the ones that are least likely to have the time to fit this into their schedules."
I was like, I don't think I can do this whole process," she says. Which is why I felt like probably a lot more women would do it if it was shorter, easier, and cheaper, right?
That experience is what led Randenkovic, a Forbes 30 Under 30 winner , to push Gameto to work on a better process and, a year later, to try it herself.
During the study, Randenkovic paid special attention to how well Gameto's solution fit with work and her time on the entrepreneurial circuit, giving talks and leading longevity seminars. In a phone interview, she ticked off a list of the downsides she encountered: one teary, emotional 24 hours when she skipped meetings and one afternoon of constipation brought on by drugs. There was also the hospital procedure in which a doctor used a probe to scrape off the immature eggs, which involved anesthesia and caused a painful next ovulation.
So half a day off work and one day where I would say my productivity at work was not optimal," she tallies. This is why we think that this technology for reducing IVF from two weeks, high cost, and medical risks to something you can do over the weekend is a big breakthrough."
Outside experts are far more cautious-and at least one has chided Radenkovic for making exaggerated claims. She told the New Yorker magazine she imagined egg retrieval could eventually be done at an egg-freezing kiosk." But for now, Gameto's process still involves some drugs and injections, so it's a hybrid of in-body and in-the-lab egg ripening.
What's more, in vitro egg maturation is not a new idea-it's been studied since the 1940s and some IVF clinics use versions of it, most often for patients with medical issues that prevent them from taking a full round of hormones. Michel De Vos, medical director at BrusselsIVF, a clinic in Belgium, estimates that lab maturation is used in fewer than 1 in 20 cases of IVF.
The reason it's not more widely used is that it's just less effective at making babies-about 35% less. That's because the procedure, as practiced today, tends to yield fewer eggs, and those eggs are also less likely to successfully develop into an embryo.
Gameto's system does look promising," according to De Vos. He says it handily beats standard methods for in vitro maturation and matches other innovative techniques in development. But he says it still won't beat standard IVF, which matures eggs in a person's body. I think we need to close the efficiency gap before we can talk about widespread application," says De Vos. There are still many steps to be accomplished before this system can be used on a large scale."
If it does improve, though, De Vos can definitely see a market for it among women who freeze their eggs to preserve a chance of getting pregnant later. That includes about 24,000 women a year in the US. A roughly similar number agree to sell their eggs so other couples can use them-donors who De Vos calls young women willing to undergo egg retrieval and get some money," adding that the hardest part is two weeks of hormone injections."
For either group, a simplified process could be attractive. Convenience. That is what's interesting," says De Vos.
That's also one reason Radenkovic is betting that Gameto's technology will be influential" in the growing egg freezing market. As a woman, when you're undergoing IVF, you want a baby then. So you have a stronger desire to go through a difficult process. And often, not always, you have a partner who's helping both financially and emotionally. So you're kind of going to put up with it," she says. Whereas if you're egg freezing ... it's to keep options open."
Woolly mammothsThe company's technology was initially developed in a Harvard University laboratory led by the geneticist George Church. Researchers there had been devising methods of quickly turning stem cells into any other cell type, often in just a few days. The trick was to add extra genes that, when turned on, would impose a developmental program on the cells, causing them to become, say, nerve or heart cells.
Church and his students were particularly interested in making eggs. If human eggs could be directly constructed in the lab, it would theoretically allow researchers to make them for all patients, no matter their age-basically solving the problem of ovarian age.Equally important to Church was a subplot then unfolding in his lab, in which a student had begun introducing woolly mammoth genes into elephant cells. He wanted to re-create the extinct pachyderm, but to do that, the project would need potentially thousands of elephant eggs. And the only way to get them would be to manufacture them.
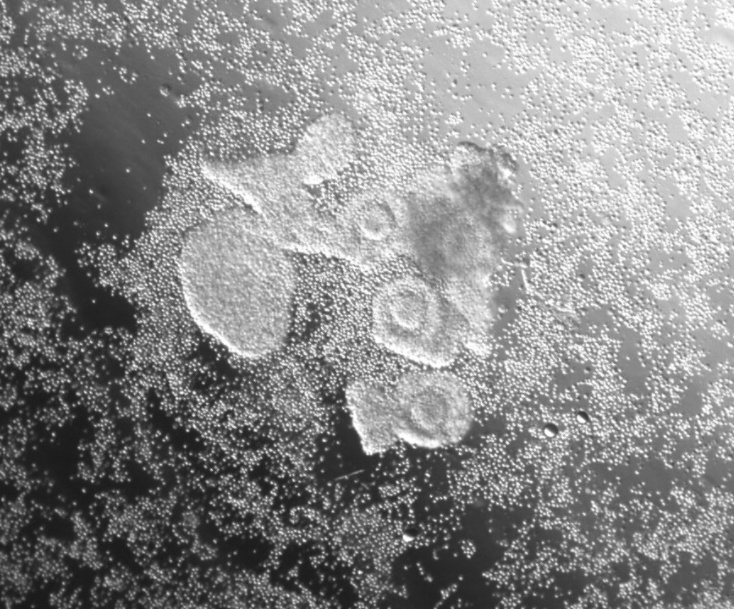 Eggs wrapped in protective tissue with lab-made granulosa cells appearing around them. CHRISTIAN KRAMME/GAMETO
Eggs wrapped in protective tissue with lab-made granulosa cells appearing around them. CHRISTIAN KRAMME/GAMETOBut making mammalian eggs has turned out to be a hard problem. It's been done in mice, but not yet in any other species. (We're still trying," Church told me.) Part of the difficulty is the sheer size of eggs, which are about 8,000 times bigger than a white blood cell. Instead, by 2022, the lab was finding success using stem cells to manufacture other components of the ovary, in particular granulosa cells-tissues in follicles that emit estradiol and play a key role in sending maturation signals to the egg.
That turned out to be the technology Gameto needed to mature eggs in a dish, and so the company licensed patent rights from Harvard and also hired one of Church's students, Christian Kramme, to lead its science efforts and become its vice president of cell engineering. (Patent rights involving elephants, kangaroos, and other nonhuman mammals went to a different startup, Colossal Biosciences, which intends to re-create several extinct species.)
Radenkovic says the company's product, which it calls Fertilo, will essentially be a tube of frozen granulosa cells that can be sprinkled around an egg to help it develop. In the paper published last week in Human Reproduction, they reported that adding these cells to a petri dish had significant positive effects on eggs, causing more of them to mature successfully. Photographs of the process show egg complexes (so called because they are still wrapped in protective tissue) with the granulosa cells appearing around them like small punctuation marks. Although the details of how it works aren't entirely clear, it appears that molecular cross talk between these supporting cells and eggs helps them finish their maturation in an organized manner.
Baby in the worksIn Gameto's study, some of the eggs collected were also fertilized with sperm from a donor bank to test their potential to make embryos. Because some of those eggs belonged to Radenkovic, I asked if she had any personal feelings toward the embryos. While only balls of a few hundred cells, they were, technically, her offspring, and they were later destroyed. Radenkovic didn't answer my question directly, but she agreed there was a weighty issue here. She said it was about managing possible harms and benefits. The company absolutely needed to demonstrate the embryos were normal, according to a battery of tests. Without that information, it would not be able to proceed to the next step: making a baby. At the same time, she says, they made as few embryos as they could. That part of the experiment was stopped as soon as the data collected cleared the bar of statistical significance.
Now we feel a lot more comfortable," she says. It's so that this would not pose a risk to a mother or her offspring."
Other methods of in vitro maturation haven't been shown to have any ill effect on children born from it. And few people seemed alarmed about Fertilo. But by adding engineered cells to the mix, Gameto has raised some new questions.Paul Knoepfler, a stem-cell scientist at University of California, Davis, said he would be concerned about unexpected changes to the eggs' epigenome, the pattern of molecular controls on our genes that get partly reset during fertilization. Embryos produced in this way may seem okay, but they may not actually be okay," says Knoepfler. Epigenetic alterations could cause health problems far down the road."
However, in the IVF industry, there's no real way to see what happens other than making babies. At some point, to determine if the method is really safe, you'd have to just forge ahead and try it in people despite the uncertainties," Knoepfler says.
That is what is likely to occur very soon. Radenkovic says the company has begun discussions with the US Food and Drug Administration about what studies may be needed to get the product approved for sale in the US. In the meantime, however, it's working with doctors outside the US. One of those overseas centers is the Concebir-Pranor clinic in Peru. It played a role in Gameto's egg study and now plans to try for live births using eggs treated with Fertilo.
We have consented patients and we're determining if they meet the criteria of the protocol," clinic doctor Silvia Ortiz and embryologist Luiz Guzman said in an email to MIT Technology Review. We plan to do the first transfers towards the end of this year."
Radenkovic had one more surprise to share: her own baby news. It had nothing to do with the company's experiment, but early this summer she learned she was pregnant. It happened the old-fashioned way. She's in her second trimester now. I am obviously excited about it. I am going to be juggling that and the CEO role," she says. I want to be that female voice that allows women not to make this compromise between career and children."