A Battery That's Tough Enough To Take Structural Loads
Batteries can add considerable mass to any design, and they have to be supported using a sufficiently strong structure, which can add significant mass of its own. Now researchers at the University of Michigan have designed a structural zinc-air battery, one that integrates directly into the machine that it powers and serves as a load-bearing part.
That feature saves weight and thus increases effective storage capacity, adding to the already hefty energy density of the zinc-air chemistry. And the very elements that make the battery physically strong help contain the chemistry's longstanding tendency to degrade over many hundreds of charge-discharge cycles.
The research is being published today in Science Robotics.
Nicholas Kotov, a professor of chemical engineer, is the leader of the project. He would not say how many watt-hours his prototype stores per gram, but he did note that zinc air-because it draw on ambient air for its electricity-producing reactions-is inherently about three times as energy-dense as lithium-ion cells. And, because using the battery as a structural part means dispensing with an interior battery pack, you could free up perhaps 20 percent of a machine's interior. Along with other factors the new battery could in principle provide as much as 72 times the energy per unit of volume (not of mass) as today's lithium-ion workhorses.
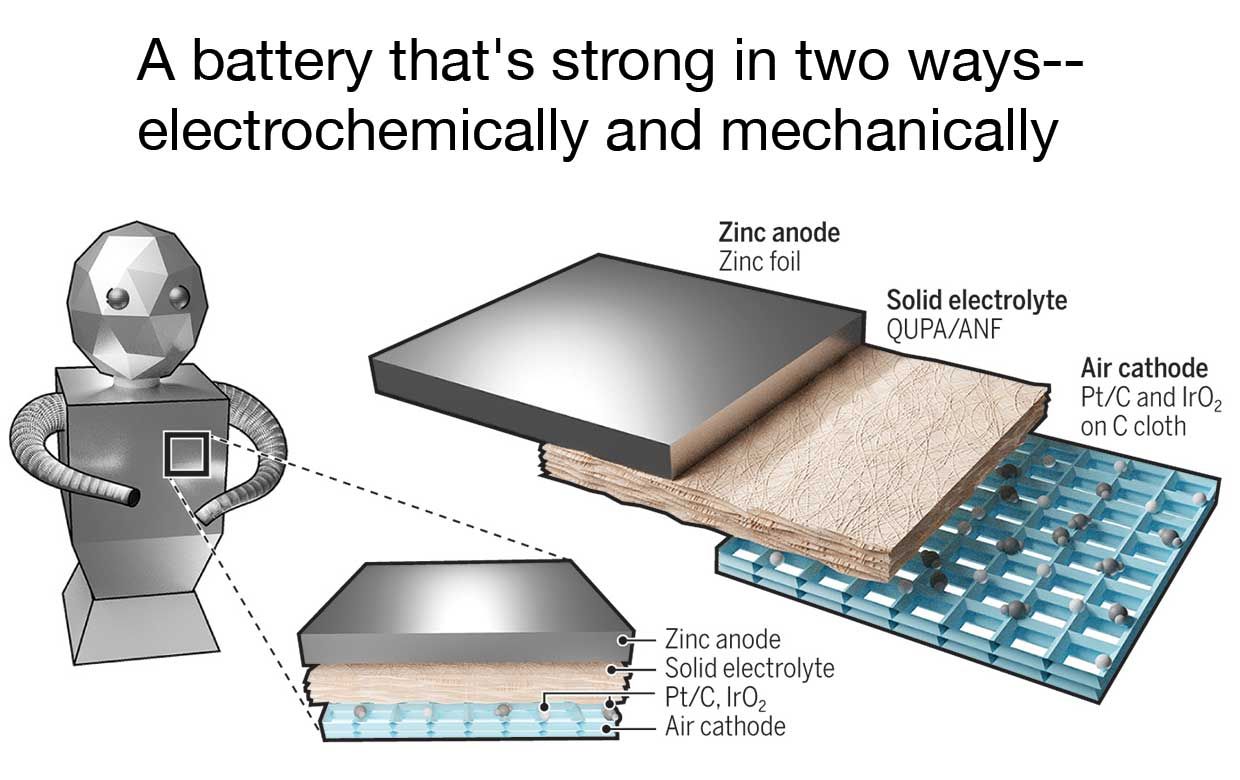 Illustration: Alice Kitterman/Science Robotics
Illustration: Alice Kitterman/Science Robotics It's not as if we invented something that was there before us," Kotov says. "I look in the mirror and I see my layer of fat-that's for the storage of energy, but it also serves other purposes," like keeping you warm in the wintertime. (A similar advance occurred in rocketry when designers learned how to make some liquid propellant tanks load bearing, eliminating the mass penalty of having separate external hull and internal tank walls.)
Others have spoken of putting batteries, including the lithium-ion kind, into load-bearing parts in vehicles. Ford, BMW, and Airbus, for instance, have expressed interest in the idea. The main problem to overcome is the tradeoff in load-bearing batteries between electrochemical performance and mechanical strength.
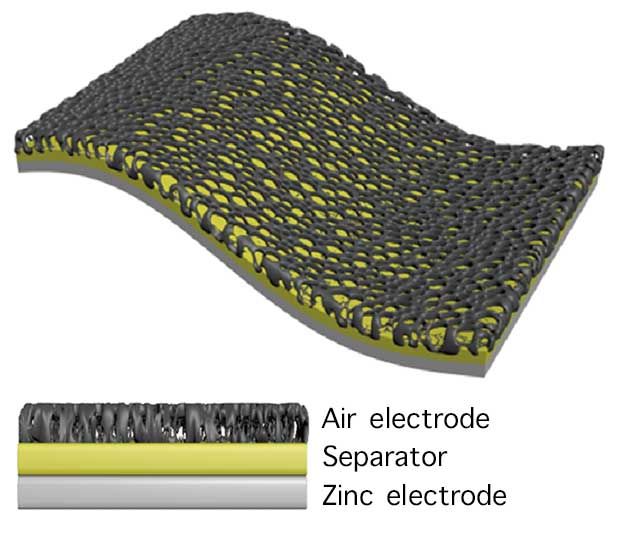 Image: Kotov Lab/University of Michigan Key to the battery's physical toughness and to its long life cycle is the nanofiber membrane, made of Kevlar.
Image: Kotov Lab/University of Michigan Key to the battery's physical toughness and to its long life cycle is the nanofiber membrane, made of Kevlar. The Michigan group get both qualities by using a solid electrolyte (which can't leak under stress) and by covering the electrodes with a membrane whose nanostructure of fibers is derived from Kevlar. That makes the membrane tough enough to suppress the growth of dendrites-branching fibers of metal that tend to form on an electrode with every charge-discharge cycle and which degrade the battery.
The Kevlar need not be purchased new but can be salvaged from discarded body armor. Other manufacturing steps should be easy, too, Kotov says. He has only just begun to talk to potential commercial partners, but he says there's no reason why his battery couldn't hit the market in the next three or four years.
Drones and other autonomous robots might be the most logical first application because their range is so severely chained to their battery capacity. Also, because such robots don't carry people about, they face less of a hurdle from safety regulators leery of a fundamentally new battery type.
And it's not just about the big Amazon robots but also very small ones," Kotov says. Energy storage is a very significant issue for small and flexible soft robots."
Here's a video showing how Kotov's lab has used batteries to form the exoskeleton" of robots that scuttle like worms or scorpions.