Researchers Are Getting Close to a Here-and-Now COVID-19 Test
 Illustration: StoryTK
Illustration: StoryTK Diagnostic testing for COVID-19 infections is one of the linchpins of the global effort to combat the deadly pandemic. But the strategy normally used for that has a few downsides. For one, the nasopharyngeal swabbing required demands the services of a health care worker, who is then put at risk of contracting the disease. Also, the sample-taking procedure, which involves sticking a very long flexible swab through a nostril and into the nasopharynx at the back of the nose and throat, is so unpleasant that some people resist being tested. And because the samples must normally be sent to a distant lab for processing, it often takes hours to days for results to become available.
What the world desperately needs is a way to diagnose infections with the virus more quickly and easily. Breakthroughs here would allow for truly widespread testing and the identification of people with asymptomatic infections, who often inadvertently spread the disease.
Researchers across the world have been racing to develop better COVID-19 tests, and biochemists and molecular biologists have made significant progress in just a handful of months. But engineers, too, have been working on technologies that might provide what everyone so dearly wants: inexpensive tests that don't require swabbing and that can be rapidly performed by anyone, anywhere-if not for this pandemic, then perhaps in time for the next one.
A Rising TideThe United States, India, Turkey, and Italy lead the world in the number of tests for COVID-19 that have been performed.
 Source: OurWorldInData.org/coronavirus
Source: OurWorldInData.org/coronavirus 16 Jan German researchers first develop a test for the COVID-19 virus.6 Feb The U.S. Centers for Disease Control starts shipping its own test kits for the COVID-19 virus.3 Aug The U.K. government announces that it will do a large-scale rollout of two new desktop COVID-19 testing systems, one from DnaNudge and one from Oxford Nanopore.26 Aug Abbott Laboratories receives emergency-use authorization from the U.S. Food and Drug Administration for its rapid BinaxNOW Ag Card test for the COVID-19 virus.
In the usual method for diagnosing COVID-19 infections, the nasopharyngeal swab sample is sent to a lab. There, technicians use a procedure called reverse-transcription polymerase chain reaction (RT-PCR) to check for the presence of the SARS-CoV-2 virus, which causes COVID-19. The processing requires first converting a characteristic segment of viral RNA into DNA and then amplifying" that DNA through a sequence of biochemical tricks and heating and cooling cycles. The presence or absence of large quantities of amplified DNA reveals whether the virus was present in the original sample.
But there are faster ways to test for infections. Perhaps the best-known example of a rapid test for this coronavirus, at least in the United States, is one being used at the White House: a COVID-19 testing system from Abbott Laboratories, which received emergency-use authorization from the U.S. Food and Drug Administration in March. It uses a toaster-size instrument called ID NOW, which Abbott introduced in 2014 to detect influenza, strep A, and respiratory syncytial virus. The device employs a biochemical strategy to amplify viral RNA without the need for heating and cooling, which allows it to produce results from a swab sample in 13 minutes or less; that's much faster than RT-PCR-based tests, which take an hour or more to run through the necessary series of thermal cycles.
The speedy results you can get with Abbott's ID NOW COVID-19 test make it immensely attractive, but there has been considerable controversy about the reliability of those results. A study posted to a preprint server in May found that the ID NOW test missed a third of positive samples, although Abbott has stated that it misses only a few percent of positives in real-world situations.
Other companies have developed similarly compact systems that can detect the presence of genetic material specific to the SARS-CoV-2 virus on-site. In August, the U.K. government announced that it would be rolling out equipment devised by two such companies, DnaNudge and Oxford Nanopore Technologies, to labs, hospitals, and nursing homes. Both machines can be operated by someone who's not a trained health professional, and both companies' tests take under 90 minutes to produce results.
In another key development, the FDA has granted emergency-use authorization for several testing protocols that do away with the nasopharyngeal swab and rely instead on saliva. These include one called SalivaDirect from the Yale School of Public Health and another developed at Rutgers University that allows people to collect the sample at home. Such advances make it more likely that spit sampling will one day become the norm for tests based on RT-PCR.
It may prove impossible, though, to create a system that amplifies genetic material of the virus rapidly, with inexpensive portable equipment, and with sufficient sensitivity to detect the virus in saliva. Fortunately, there is a fundamentally different strategy that might check all these boxes: antigen-based tests.

 Photos: Abbott In The Cards: Abbott Laboratories' new antigen-based test is performed on a card that's about the size of a credit card [top]. A nasal swab is inserted into the card, a reagent is added, and 15 minutes later, a technician reads the result and uses a smartphone app to provide the person tested with a time-stamped verification of their results [bottom].
Photos: Abbott In The Cards: Abbott Laboratories' new antigen-based test is performed on a card that's about the size of a credit card [top]. A nasal swab is inserted into the card, a reagent is added, and 15 minutes later, a technician reads the result and uses a smartphone app to provide the person tested with a time-stamped verification of their results [bottom]. Antigen-based tests don't look for genetic material specific to the virus. Instead, they use antibodies-large molecules that have just the right shape to bind with protein molecules specific to the virus, such as the now-famous spike protein that decorates the surface of SARS-CoV-2. The molecular targets of such antibodies are called antigens.
When people are infected, one part of their immune response is to manufacture antibodies to the virus. These molecules can also be mass produced and incorporated into test kits to be mixed with a sample of mucus or saliva. The tricky part is determining whether the antibodies in the test solution have indeed attached to the virus, which is what signals that the person being tested has an active infection.
Biochemists have experience with this challenge and have developed various antigen-based point-of-care tests for other viral diseases. At the time of this writing, the FDA has granted emergency-use authorization to four companies for such antigen-based tests for the COVID-19 virus. One of these, developed by Becton, Dickenson and Co., takes advantage of the company's already-deployed Veritor handheld instruments and provides results in just 15 minutes.
But the world could still benefit from the ability to test people for this virus using simpler, cheaper equipment and by testing saliva, instead of requiring a swab. The best situation would be like a pregnancy test," says Angela Rasmussen, a virologist at Columbia University. Spit on a stick or into a collection tube and have a clear result 5 minutes later."
On 26 August, Abbott Laboratories obtained emergency-use authorization from the FDA for a test that is approaching that ideal, which it calls the BinaxNOW Ag Card. Unlike this company's earlier rapid-testing system, this one requires no desktop analyzer. The antigen-based test is performed on a card that's about the size of a credit card: A nasal swab is inserted into the card, a reagent is added, and 15 minutes later the technician reads the result. The only relevant device here is a smartphone.
That's because Abbott has also developed a companion phone app, which it called Navica [PDF]. People who install the app can present their phones to the technician, who will then enter the results of their tests through the Navica app on his or her own phone. Those who test negative will soon have time-stamped QR codes on their phones that attest to that result, enabling schools and workplaces to check people's statuses using the app's verification feature.
The availability of Abbott's BinaxNOW Ag Card, which the company expects to ship soon in large quantities (tens of millions each month) for a cost of only US $5 per test, portends a new phase of the pandemic, one in which it becomes much more straightforward to identify people infected with the COVID-19 virus. Going forward, we still require a test that is equally fast and inexpensive but that could work with saliva, so that anyone, anywhere could determine whether they've been infected. Here's where some new technologies might be able to make a special contribution in the quest for such a test.
At the University of Minnesota, materials-science engineer Jian-Ping Wang had been working on a novel technique called magnetic-particle spectroscopy for detecting influenza, but at the end of March he made a big pivot, modifying his device to aid in the battle against COVID-19. Wang's approach mixes the sample with magnetic nanoparticles coated with antibodies that target the SARS-CoV-2 virus. The challenge, as with any antigen-based technique, is figuring out whether the virus has indeed attached to them.
Lassoing a Virus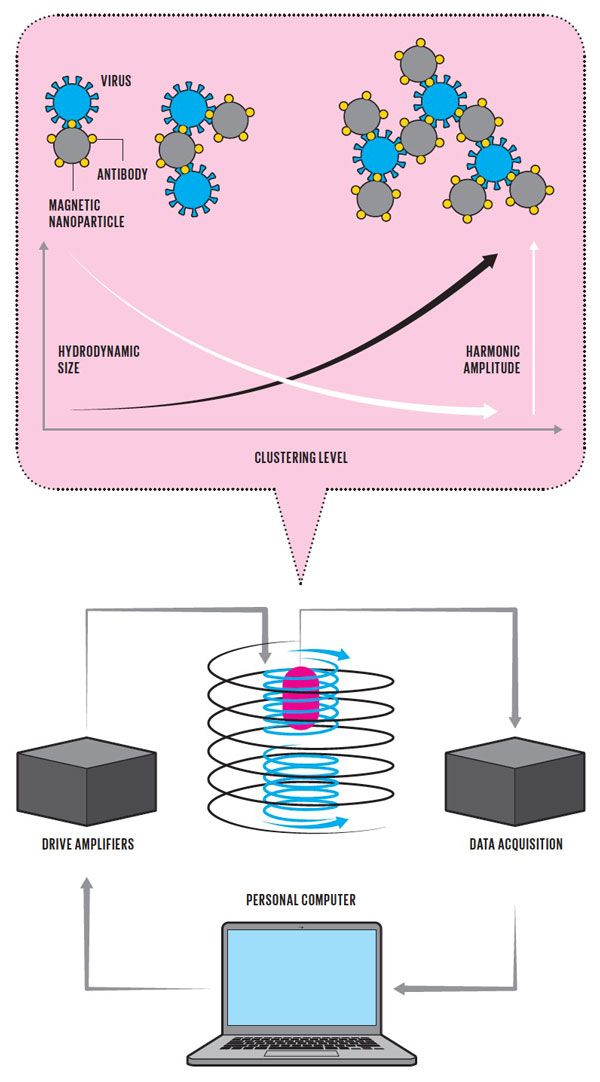 Illustration: Chris Philpot The approach to testing that Jian-Ping Wang at the University of Minnesota is taking relies on magnetic particles that are a few tens of nanometers in diameter [top, gray circles]. These particles are coated with antibodies [yellow] that can attach to certain structures on the surface of the SARS-CoV-2 virus [blue]. When mixed with a sample that contains the virus, the antibody-coated magnetic particles tend to clump together. These clumps, having a larger hydrodynamic size than individual particles, are slower to rotate into alignment with a changing magnetic field, applied using coils of wire that surround the sample, which for clarity is shown here as a single spiral [bottom, black]. This difference in agility can be detected using two counterwound sense coils [blue], which nullify the effects of the applied field while picking up signals from the changing magnetization of the sample [pink]. Low harmonic amplitude in the sensed signal indicates the magnetic particles have clumped, revealing the presence of the virus.
Illustration: Chris Philpot The approach to testing that Jian-Ping Wang at the University of Minnesota is taking relies on magnetic particles that are a few tens of nanometers in diameter [top, gray circles]. These particles are coated with antibodies [yellow] that can attach to certain structures on the surface of the SARS-CoV-2 virus [blue]. When mixed with a sample that contains the virus, the antibody-coated magnetic particles tend to clump together. These clumps, having a larger hydrodynamic size than individual particles, are slower to rotate into alignment with a changing magnetic field, applied using coils of wire that surround the sample, which for clarity is shown here as a single spiral [bottom, black]. This difference in agility can be detected using two counterwound sense coils [blue], which nullify the effects of the applied field while picking up signals from the changing magnetization of the sample [pink]. Low harmonic amplitude in the sensed signal indicates the magnetic particles have clumped, revealing the presence of the virus. The clever scheme that Wang has worked out relies on the behavior of his magnetic nanoparticles when they're subjected to an external magnetic field. Electrical engineers are well aware that the magnetization of iron-containing materials can change direction when a magnetic field is applied to them. For the 30-nanometer-diameter magnetic particles that Wang works with, that change in magnetization comes about by the physical rotation of the particle in solution. These particles are small and nimble enough to act like tiny compass needles, rotating into alignment with the applied magnetic field. If you subject them to a slowly oscillating magnetic field-say, one created inside a coil of wire-their orientations will flip back and forth in sync with the changes in the polarity of the applied field.
The surface of each magnetic particle holds more than one antibody, and the surface of each viral particle contains more than one antigenic protein. So when many of these two kinds of particles are present together in solution, they tend to link up into extended networks, with the virus acting as a sort of glue holding clusters of magnetic particles together.
Those clumps, being much larger than single magnetic particles, cannot rotate as quickly in solution, so they behave differently in an oscillating magnetic field. As the frequency of the applied field grows into the kilohertz range, the rotation of the particle clusters increasingly lags behind the applied field. You'd see something analogous if you placed a compass inside a coil and applied a current that oscillated in polarity faster than the needle could physically respond to the rapid changes in the magnetic field.
It's difficult to measure directly how much the rotating magnetic particles lag the applied field. But for complicated reasons, when the lag is small, the oscillatory motions of the magnetic particles include frequencies that were not present in the applied field. Conversely, when the lag is substantial, those additional frequency components are much diminished. So the frequency content provides a simple measure of whether or not clusters formed. If they did form, it indicates that the target virus was present.
The detection of those changes in the frequency content is not difficult, requiring only a suitable pickup coil, an amplifier, and a computer to do some straightforward digital signal processing. Wang is using a benchtop system connected to a laptop now, but he envisions all this being done by a handheld device. After the test sample mixes with the magnetic nanoparticles for about 10 minutes, it would be placed in that device, which in a few seconds would provide a readout of whether the virus was present. We're screening different technologies to figure out the best way to create a low-cost and convenient [device] for the customer to use," says Wang.
We can certainly hope that Wang will be able to lower the costs enough for his device to become a consumer product and that it will have sufficient sensitivity to detect the COVID-19 virus in saliva. But his isn't the only possible solution on the table.
At the University of Pennsylvania's Penn Center for Research on Coronavirus and Other Emerging Pathogens, Ping Wang (no relation to Jian-Ping Wang at the University of Minnesota) is working on yet another new way to detect tiny quantities of the SARS-CoV-2 virus. Wang calls the technique her group has pioneered a microbubbling digital assay." It also uses magnetic particles to which SARS-CoV-2-specific antibodies are attached. But these magnetic particles are much larger than those used in the other Wang's magnetic-particle spectroscopy: Rather than being tens of nanometers in diameter, these are a few micrometers across. Her approach also employs nanometer-size particles of platinum that are attached to the same antibodies.
When both of these kinds of engineered particles are added to a solution containing SARS-CoV-2, the virus hooks up to both, sometimes linking a platinum nanoparticle on one side to a much larger magnetic particle on the other. The challenge is to figure out whether such a virus sandwich has formed. The tool Wang devised to determine that is a novel microchip. Being made of various polymers, it's very different from the silicon chips found in electronic gadgets. But like electronic chips, it's fabricated using lithography and physical vapor deposition.
Wang's chip is about 3 millimeters on a side. It contains an array of tiny square depressions, each 14 micrometers wide and 7 micrometers deep, which is just large enough to hold one of those virus sandwiches. A magnet is placed beneath the chip, and hydrogen peroxide is added to the sample solution on top. The magnet draws the magnetic particles downward, and some land in the tiny depressions.
This chip can reveal the presence of a virus because platinum catalyzes the decomposition of hydrogen peroxide into water and oxygen. Any virus sandwich caught in a well will generate oxygen there. Virus sandwiches that land between wells don't form bubbles for reasons that are currently unclear. But over time, the oxygen created inside a well forms a bubble, which is big enough to be seen with a smartphone camera using just a small amount of magnification. If there is no virus present in the sample, the platinum nanoparticles won't be linked up with the magnetic particles, and no bubbles will be generated inside the wells.
 Images: University of Pennsylvania/Wiley Seeing Is Believing: Researchers at the University of Pennsylvania explored the intrinsic sensitivity of their microbubbling assay by taking smartphone images of their chip after exposing it to different concentrations of platinum nanoparticles. The number of bubbles produced grows with the increasing concentration of those particles. The researchers also showed that the number of bubbles grows with the increasing concentration of the targeted protein in the sample.
Images: University of Pennsylvania/Wiley Seeing Is Believing: Researchers at the University of Pennsylvania explored the intrinsic sensitivity of their microbubbling assay by taking smartphone images of their chip after exposing it to different concentrations of platinum nanoparticles. The number of bubbles produced grows with the increasing concentration of those particles. The researchers also showed that the number of bubbles grows with the increasing concentration of the targeted protein in the sample. To automate the task of bubble identification and counting during her work on this technique last year, Wang trained a neural network using hundreds of images. That allowed her to develop a smartphone app that is able to tally the number of bubbles on a chip in seconds.
Currently, Wang's team is trying to miniaturize the equipment needed for this microbubbling assay, so that it could be carried out using a device attached to a smartphone. She's now testing this approach using samples from COVID-19 patients and planning to apply for emergency-use authorization from the FDA if the system proves accurate enough.
It's too soon to know whether either of these two new antigen-detection technologies will indeed provide a way to detect the COVID-19 virus with inexpensive equipment, in near real time, and with sufficient sensitivity to do so using a person's saliva. With luck, one of them will meet that high bar. With even more luck both will, which would help enormously when it comes time to scale up production of the requisite devices to meet demand. One thing is for sure, though: If scientists and engineers are able to solve this technical challenge in the not-too-distant future, many people around the world will rapidly incorporate such tests into their daily lives.
This article appears in the October 2020 print issue as A Here-and-Now COVID-19 Test."