This Is How We’ll Vaccinate the World Against COVID-19
 Photo-illustration: Edmon de Haro
Photo-illustration: Edmon de Haro In a triumph of science, the first two large-scale trials to report the effectiveness of vaccines against SARS-CoV-2-the deadly, highly contagious virus that causes COVID-19-were both great successes right out of the gate. In November, the pharmaceutical giant Pfizer and the much younger biotech company Moderna both reported that their vaccines were about 95 percent effective in preventing cases of COVID-19. The news came just 10 months after the virus was first isolated and sequenced in a lab in China.
As of early December, 50 other candidate vaccines were making their way through human clinical trials, according to the World Health Organization. Thirteen of those vaccines were already in the final stage before approval, each being tested on tens of thousands of volunteers to check for side effects and measure efficacy: how well the shots protect against the disease. One of those, made by AstraZeneca and the University of Oxford, also showed promising-though less clear-efficacy results in late November.
But even before those vaccines neared the finish line, the heaviest burdens of ending the pandemic and restoring the global economy had shifted from the scientists to the engineers. Our hopes now hinge on the technologists who are challenged with manufacturing and transporting billions of doses of new, highly complex biotech products-and the public health officials figuring out how best to distribute them to a world that can hardly wait.
Throughout 2020, vaccine producers and their suppliers constructed new factories and otherwise increased their capacity while governments, international agencies, and philanthropies signed billion-dollar contracts, preordering doses by the hundreds of millions. In the United States, the federal initiative known as Operation Warp Speed deployed a budget of more than US $12 billion to develop, test, and mass-produce new vaccines along with the vials, syringes, and other materials needed to deliver them to an anxious populace.
Advance Purchases by Operation Warp Speed| Vendor | Contract value | Procurement |
|---|---|---|
| Sanofi/GlaxoSmithKline | US $2.1 billion | 100 million doses of its protein-based vaccine, with an option to buy another 500 million doses |
| Pfizer/BioNTech | $1.95 billion | 100 million doses of their RNA-based vaccine, with an option to buy another 500 million doses |
| Novavax | $1.6 billion | 100 million doses of its protein-based vaccine |
| Moderna | $1.5 billion | 100 million doses of its RNA-based vaccine, plus an option to buy 400 million more doses |
| AstraZeneca | $1.2 billion | 300 million doses of its viral vector vaccine |
| Johnson & Johnson | $1 billion | 100 million doses of its viral vector vaccine, plus an option to buy another 500 million doses |
| Emergent BioSolutions | $628 million | Expanded manufacturing capacity for Johnson & Johnson, Novavax, and Vaxart vaccines |
| Fujifilm and Texas A&M University | $265 million | Manufacturing capacity to produce Novavax vaccine or another vaccine |
| Corning | $204 million | Expanded manufacturing capacity for 164 million vials per year and priority access to Valor glass vials |
| Grand River Aseptic Manufacturing | $160 million | Aseptic fill-and-finish manufacturing capacity |
| SiO2 Materials Science | $143 million | Expanded capacity for 120 million glass-coated vials per year |
| ApiJect Systems America | $138 million | 100 million prefilled syringes; expanded manufacturing capacity for more than 500 million prefilled syringes in 2021 |
| Cytiva | $31 million | Expanded supply of cell culture media, mixer bags, and FlexFactory bioreactors |
Moncef Slaoui, the initiative's chief scientist, told IEEE Spectrum in October that the U.S. government had already begun stockpiling two vaccines (from Pfizer and Moderna), and that commercial-scale production was beginning on two others. So if and when they are approved" by regulators at the U.S. Food and Drug Administration (FDA), he said, those can be used in the [U.S.] population immediately."
Creation and deployment of a new vaccine against a novel disease normally takes at least a decade. The audacious goal of Operation Warp Speed and like-minded efforts in other nations is to complete this feat in less than two years. The pace is every bit as intense as the space race of the 1960s, but the stakes are far higher.
There are plenty of reasons for skepticism. When was the last time anybody made a billion of anything safely and reliably?" asks Arthur Caplan, a bioethics professor at NYU Grossman School of Medicine. Never," he says. Plants go offline, crap breaks, you can't find a part." Caplan argues that we should expect snafus: There's a ton of things that can go wrong just on manufacturing."
But also consider this: In 2019, brewers in the United States used applied microbiology to ferment, filter, fill, package, and distribute nearly 50 billion bottles and cans of beer-all in copacetic single-dose units, most of it refrigerated.
Will the university, industry, and government teams grappling with the vaccine challenge be able to bring together the interrelated technical systems that must work in concert-including massive bioreactors and purification lines, acres of fast-fill vials, and thousands of planeloads of ultracold shipping containers? Can humanity really pull this off?
Somewhat surprisingly, the answer so far appears to be: Yes, we can.
What's NextMass-producing a safe and effective vaccine is just the beginning of a global challenge
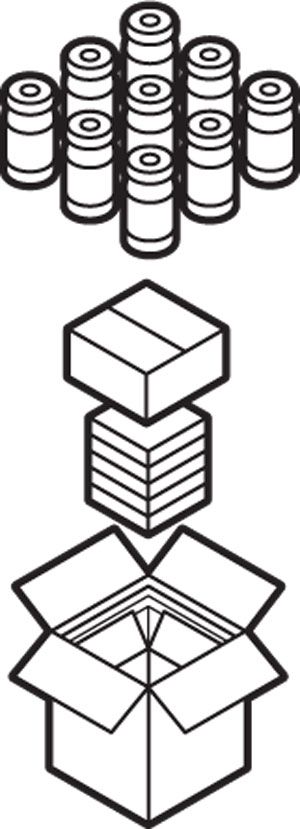 Illustrations: Chris Philpot Packaging The vaccine must be poured into millions of glass vials, and those vials must be packed into custom containers.
Illustrations: Chris Philpot Packaging The vaccine must be poured into millions of glass vials, and those vials must be packed into custom containers.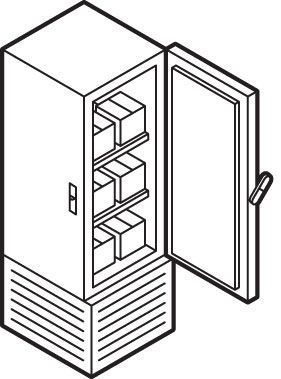 Cooling Most vaccines must be kept at between 2 C and 8 C; RNA vaccines must be frozen at much lower temperatures.
Cooling Most vaccines must be kept at between 2 C and 8 C; RNA vaccines must be frozen at much lower temperatures.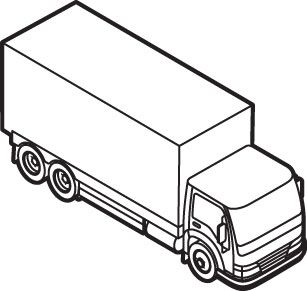 Transport Containers must move across the world via planes and trucks, while sensors keep track of their temperature and location.
Transport Containers must move across the world via planes and trucks, while sensors keep track of their temperature and location. Distribution Doses must be quickly doled out to the hospitals, clinics, and pharmacies that have the facilities to store them and the infrastructure to administer them.
Distribution Doses must be quickly doled out to the hospitals, clinics, and pharmacies that have the facilities to store them and the infrastructure to administer them.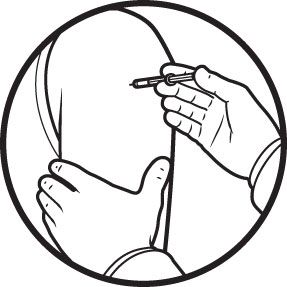 Vaccination As the leading COVID-19 vaccines require two doses for full protection, people must be tracked and brought back to the clinic after several weeks.
Vaccination As the leading COVID-19 vaccines require two doses for full protection, people must be tracked and brought back to the clinic after several weeks.
Not everything will go smoothly. Paul Offit, a member of the COVID-19 vaccine working group at the U.S. National Institutes of Health, sat down in June to talk with the editor of the Journal of the American Medical Association about the steep road ahead. The hardest thing about making a vaccine is mass-producing it," Offit said. You have to have the right buffering agent, the right stabilizing agent. You have to have the right vial. You have to do real-time stability studies to make sure that when the vaccine leaves the manufacturing plant, that the time it takes to get from the tarmac to the person's arm does not cause any problems. Because, remember, when you're shipping vaccines, they're going to be exposed to high temperatures and low temperatures, and you have to make sure that you have a stable product."
Take, for example, the RNA-based vaccine that Pfizer and its German partner BioNTech developed-the first to be approved by the FDA. This kind of vaccine contains slightly altered pieces of the virus's genetic material (RNA) encased in nanometer-size fatty blobs, which fuse with human cells and cause them to produce the SARS-CoV-2 spike protein, thus triggering an immune response in the body. None of the vaccine experts interviewed for this article had dared to hope that any COVID-19 vaccine-let alone an RNA-based vaccine, a type that's never before been commercialized-would achieve a 95 percent efficacy rate.
But that stellar effectiveness can wink out if the vaccine gets too warm for too long. As Offit emphasized, temperature affects all vaccines; most (including AstroZeneca's) must remain between 2 C and 8 C to retain potency. RNA vaccines, however, are especially unstable.
At its assembly plants in Kalamazoo, Mich., and Puurs, Belgium, Pfizer has warehouses full of ultracold freezers to store its vaccine at -70 C. Workers pack the frozen vials into custom-built containers that each hold about 1,000 of them, along with a layer of dry-ice pellets. Also in the box is a GPS-enabled thermal sensor that transmits the temperature and location of the package as it moves via trucks and planes to distribution centers throughout the world.
Distributors are rapidly scaling up too. UPS has said that it's building two warehouses full of deep freezers-one in Louisville, Ky., and another in the Netherlands-that are capable of storing enough COVID-19 vaccines to inoculate millions of people. FedEx, which routinely delivers about 500,000 dry-ice-packed shipments a month, is doing the same in Memphis, Indianapolis, and Paris.
Rich Gottwald, president of the Compressed Gas Association, says that a nationwide shortage of carbon dioxide last spring spurred CO2 producers to work closely with vaccine makers, ensuring that dry ice will be there when and where they need it. There may be some challenges in getting the vaccine distributed, but dry ice is not one of those challenges," he says.
Most of these trips from factory to pharmacy or clinic should take no more than three days, and Pfizer's vaccine stays fresh for up to 10 days in its container when unopened. Once thawed, the liquids must be kept in pharmacy-grade refrigerators and used within five days. Moderna claims its RNA vaccine can be transported and stored in deep freezers at -20 C for up to six months and then refrigerated at distribution points for up to 30 days.
Unfortunately, only technologically advanced nations will be able to manage all these logistical complexities. In September, the shipping company DHL analyzed the transport challenges posed by a global rollout of COVID-19 vaccines. Its report concluded that mass distribution of vaccines requiring dry ice for storage will be feasible in only about two dozen countries, accounting for 2.5 billion people. All of Africa, most of South America, and much of Asia would struggle to put such a vaccine to widespread use.
In contrast, DHL estimates, around 60 countries would find it quite possible to inoculate their combined 5 billion residents with vaccines like AstraZeneca's, which can be stored and transported at refrigerator temperatures of 2 C to 8 C (a typical temperature in pharmaceutical supply chains). Both ease of transport and substantially lower manufacturing costs favor more traditional vaccines, such as those that use harmless viruses to trigger an immune response. AstraZeneca's vaccine, for example, is expected to sell for about a third the cost of the RNA vaccines.
In the hope of making coronavirus vaccines available to even the poorest nations, the World Health Organization, the Coalition for Epidemic Preparedness Innovations, and Gavi, the Vaccine Alliance have joined together to form the COVAX initiative. The coalition has been raising money to secure 2 billion vaccine doses through 2021 for the 90-plus low- and middle-income countries expected to participate, many of which can't afford to buy or make vaccines on their own. As of mid-November, COVAX reported about US $2 billion in pledged donations, but it said at least $5 billion more is needed to achieve its goal.
These front-runners are just the opening salvo in what will be a protracted battle against SARS-CoV-2. Reinforcements, in the form of other vaccine options, should arrive in 2021 and will be crucial in bringing this pandemic to an end.
No one manufacturer is going to be able to scale up and make enough doses for 7 billion people," says Leonard Friedland, director of scientific affairs and public health at GSK Vaccines. So I hope they all work."
Pfizer said in July that it was aiming to produce 100 million doses of its product by the end of 2020, but by November it had halved that estimate. The hardest part for Pfizer has been mixing the synthetic pieces of RNA with fatty acids and cholesterol to form delivery particles of just the right size, says Slaoui of Operation Warp Speed. These mixing operations are very complex," he says.
And there is likely to be a shortage of cholesterol needed for the lipid nanoparticles, warns Jake Becraft, CEO of Strand Therapeutics, a biotech company in Massachusetts that is developing RNA vaccines of its own. The simple fact is that those supply chains were nowhere near ready for the demand of billions of vaccines," Becraft says. Some capacity can be redirected to support COVID-19 vaccine production, he says, but it will also come at the cost of a lot of drugs in the pipeline for diseases like cystic fibrosis and cancer" that require the same ingredients.
Nevertheless, Pfizer has projected that it will produce up to 1.3 billion doses of COVID-19 vaccine by the end of 2021. Because each person's inoculation requires two doses spaced two or three weeks apart, that should be enough to protect roughly 650 million people. The U.S. government has prepurchased 100 million of those doses, with an option to buy 500 million more.
4 Ways to Make a COVID-19 VaccineThe vaccines under development fall within four categories. The manufacturing processes shown here (in simplified form) depict the procedures of leading vaccine candidates.
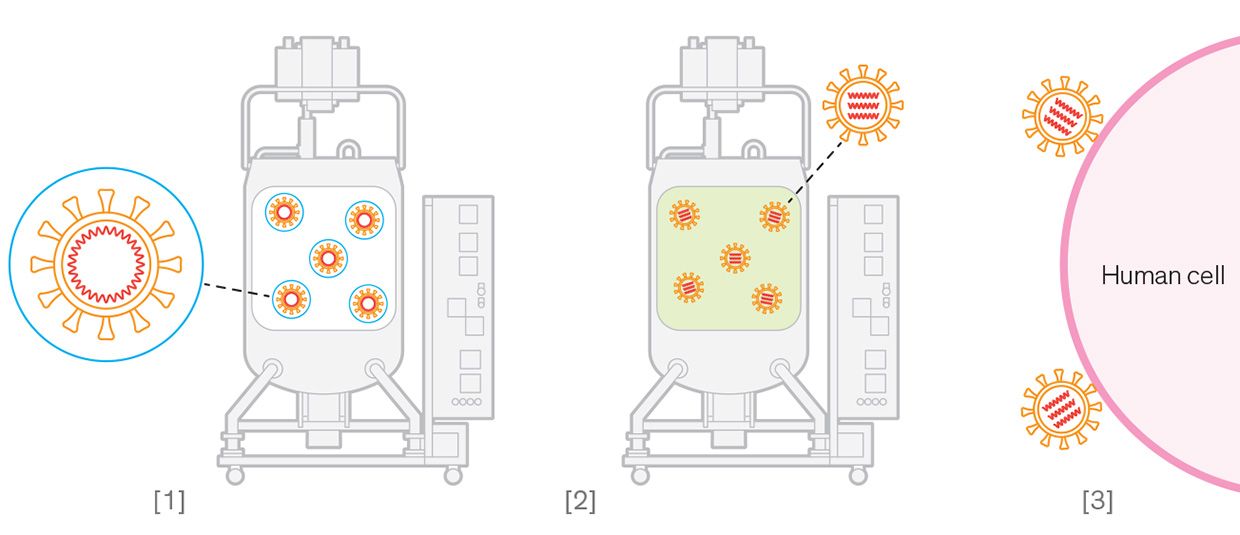 Illustrations: Chris Philpot Inactivated Virus Vaccine
Illustrations: Chris Philpot Inactivated Virus Vaccine [1] The live coronavirus is allowed to infect trillions of mammalian cells in a bioreactor. [2] The virus is extracted from the cells and then chemically treated to inactivate its RNA. [3] The inactivated virus can't replicate inside human cells, but its intact proteins trigger an immune response.
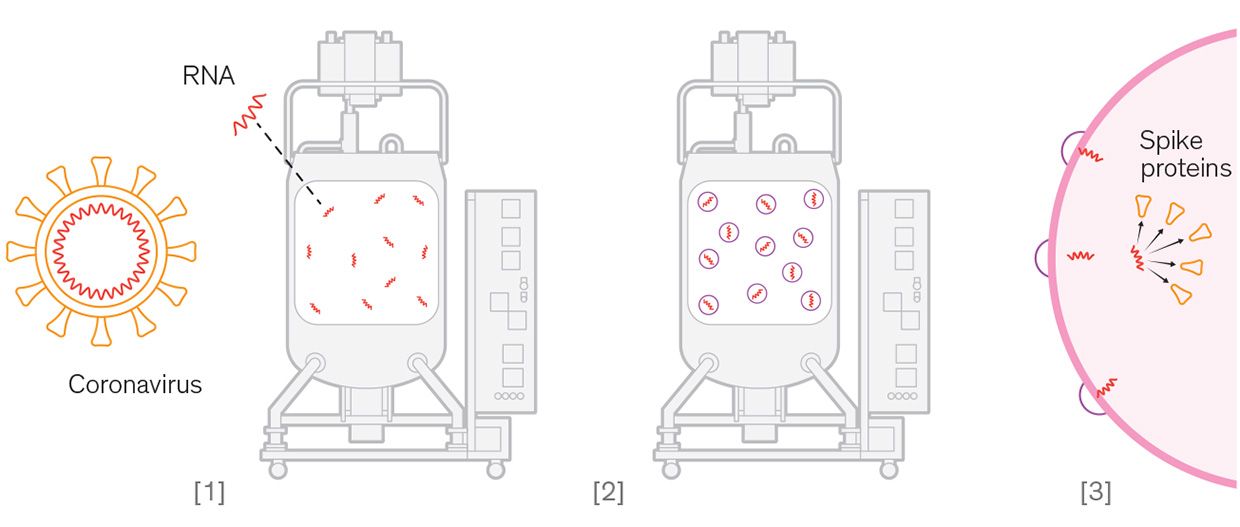 RNA Vaccine [1] A segment of coronavirus RNA that codes for production of spike proteins is synthesized. [2] The RNA segment is mixed with fatty acids and cholesterol to form lipid delivery particles. [3] The lipid particles fuse with human cells' membranes and release the RNA. The cells produce spike proteins that trigger an immune response.
RNA Vaccine [1] A segment of coronavirus RNA that codes for production of spike proteins is synthesized. [2] The RNA segment is mixed with fatty acids and cholesterol to form lipid delivery particles. [3] The lipid particles fuse with human cells' membranes and release the RNA. The cells produce spike proteins that trigger an immune response.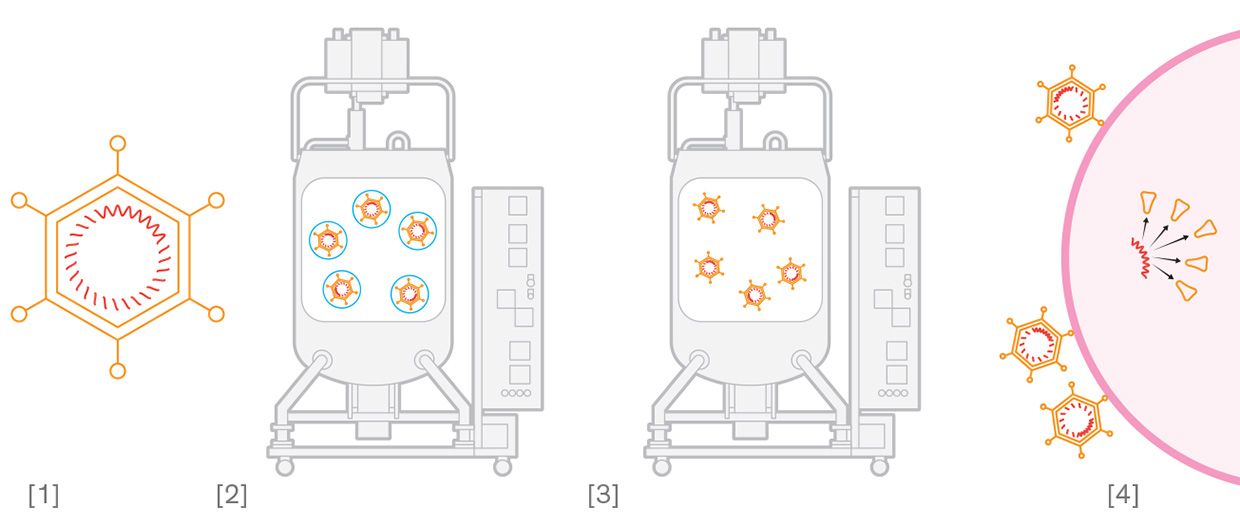 Viral Vector Vaccine [1] A harmless adenovirus is engineered to include coronavirus genetic material. [2] The engineered virus is grown in a cell culture. [3] The engineered virus is extracted from the cells and purified into a vaccine. [4] The virus can't replicate inside human cells, but it produces coronavirus spike prot eins that trigger an immune response.
Viral Vector Vaccine [1] A harmless adenovirus is engineered to include coronavirus genetic material. [2] The engineered virus is grown in a cell culture. [3] The engineered virus is extracted from the cells and purified into a vaccine. [4] The virus can't replicate inside human cells, but it produces coronavirus spike prot eins that trigger an immune response.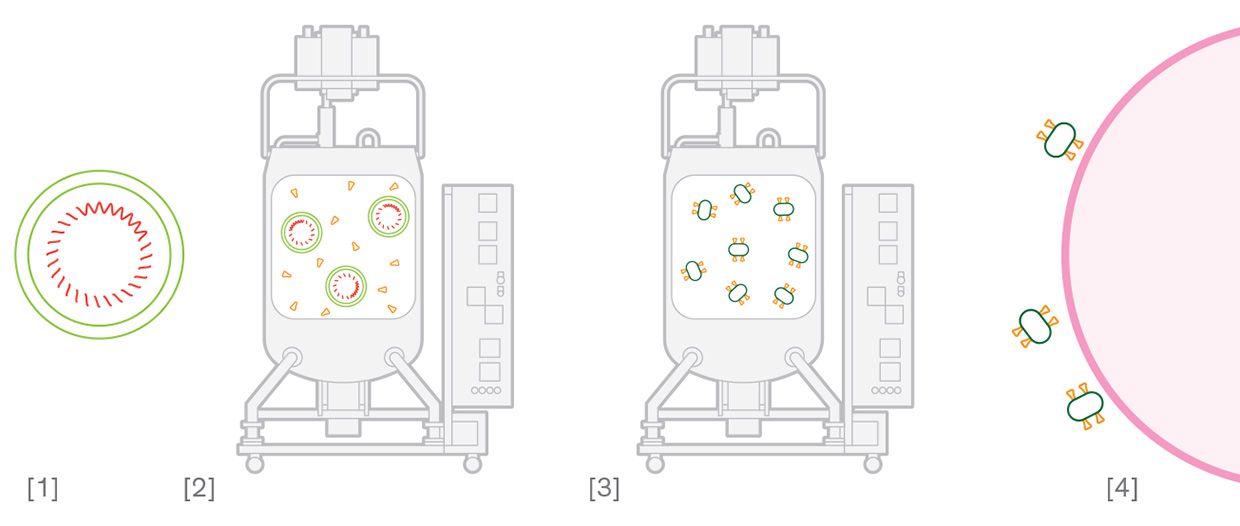 Protein Vaccine [1] An insect virus is engineered to include coronavirus genetic material. [2] The engineered virus is grown in an insect cell culture, where the virus produces the coronavirus spike proteins. [3] The spike proteins are mixed with harmless microscopic particles. [4] The particles carry the spike proteins into the body to trigger an immune response.
Protein Vaccine [1] An insect virus is engineered to include coronavirus genetic material. [2] The engineered virus is grown in an insect cell culture, where the virus produces the coronavirus spike proteins. [3] The spike proteins are mixed with harmless microscopic particles. [4] The particles carry the spike proteins into the body to trigger an immune response.
As of press time, Moderna was hoping that its vaccine would be ready for broad release to the public in late December, assuming that all went smoothly with its licensing application to the FDA. The company signed up a manufacturing partner, Lonza Group, which is scaling up global manufacturing to be able to deliver 100 million doses a year from its site in Portsmouth, N.H., and another 300 million doses a year from a larger facility in Visp, Switzerland.
Meanwhile, in China, the companies Sinopharm and Sinovac have late-stage trials underway on three vaccines that contain intact coronavirus, which is harvested from live cell cultures and then chemically treated so that it cannot reproduce inside a person. This technology, used to make the annual flu vaccine and many others, has a long track record of success. And China has lots of manufacturing capacity for making inactivated-virus vaccines, notes John Moore, a professor of immunology at Weill Cornell Medical School in New York. Sinopharm is reportedly gearing up to produce 1 billion doses of its vaccine in 2021, if the product succeeds in trials.
But drugmakers elsewhere have largely steered clear of vaccines made from live cells infected with the SARS-CoV-2 virus, which pose obvious dangers to workers. The need for biosafety level 3" facilities designed and certified to handle such biohazards makes such products harder to scale up, according to Kate Bingham, who chairs the U.K. government's Vaccines Taskforce.
Of the remaining five vaccines in final-stage trials, four (including the AstraZeneca vaccine) are made by inserting a key gene from the coronavirus into a largely harmless human or chimpanzee adenovirus. After injection, these viral vector vaccines produce the important SARS-CoV-2 protein fragment inside the body, triggering an immune reaction.
The tricky part is harvesting enough of the engineered adenoviruses from the cell cultures in which they are grown. The biggest issue as we scale up has been optimizing the infection step," Slaoui says. Stirring 2,000 liters of living cells well enough to let the virus infect most of them-but gently enough so as not to rupture many of them-has proven difficult.
A similar scale-up challenge comes up in the production of the final kind of vaccine, one made by Novavax in Gaithersburg, Md. The company makes its protein vaccine in a factory in Morrisville, N.C., by growing huge batches of armyworm moth cells, which it has genetically engineered to churn out copies of a subunit of the coronavirus's spike protein. After breaking up the cells and purifying the slurry, workers mix the desired protein with harmless microscopic particles that will carry the virus fragment into the body to trigger an immune response.
Here, Slaoui says, the big challenge is to bust up the cells in a way that doesn't completely overwhelm the purification process with unwanted moth proteins. The company has a clinical trial underway in the United Kingdom, but in late November it delayed planned trials in the United States and Mexico because production was not scaling up as quickly as anticipated.
Nevertheless, Novavax has promised the U.S. government 100 million doses as they come off its production lines, and the company claims it has the capacity at a plant it bought in the Czech Republic to make a billion more doses in 2021.
If several vaccines gain approval and begin ramping up production in parallel, could there be what engineers call a common mode" failure? The vaccines may vary, for example, but so far they're all packaged the same way-in 5-milliliter vials made of a special kind of glass-and then injected into the arm via syringe.
Syringes are probably less of a problem than vials and stoppers," says Georges Benjamin, executive director of the American Public Health Association. If I was wanting to pay attention to what can go wrong, it'd be that."
Vaccines are so potent that each vial typically holds enough for five doses. Moderna claims its RNA vaccine is stronger still, so doctors can get 10 doses from every vial. On the one hand, that means that a 1,000-vial container of Moderna vaccine could give 10,000 people one of the two doses they will need. On the other hand, every vial that breaks wastes that many more doses.
The problem with frozen vaccines isn't that ultracold temperatures make vials brittle, says Robert Schaut, the scientific director of pharmaceutical technologies at the glass-making company Corning. You're already below its glass-transition temperature, unlike a plastic or other material. So glass is exactly as strong at -70 C as it is at room temperature," he points out. But when you cool vaccine down to those temperatures, the liquid expands and puts a lot of stress on the glass."
Two years ago, Corning came out with a stronger, aluminosilicate glass that can be prestressed during vial manufacture by replacing sodium atoms in the materials with potassium atoms. That switch introduces hundreds of megapascals of compressive stress into the material-plenty enough to resist breakage during freezing or transport, Schaut says. He claims that the stronger glass vials also eliminate flaking and dramatically reduce tiny particles dislodged during the filling process, which in the past has led to recalls of conventional glass vials.
More useful still, the new vials are slippery. At the fill-finish stage of vaccine production, when big batches of vials are jostling along through the machinery, the slick coating on the vials lets them glide past each other more easily. Reducing jams on manufacturing lines adds 20 to 50 percent to the throughput, Schaut says, and once lines are moving smoothly, operators can double their speed.
Since the first quarter of 2020, Corning has been shipping millions of vials a month to its Operation Warp Speed partners from its plants outside Corning, N.Y. The company used part of its $204 million government contract to speed construction of a new factory in North Carolina, set to come online next year. Schaut says Corning should now be able to churn out 164 million vials a year-enough to ship at least 820 million doses of vaccine.
We set the objective to have enough vaccine to immunize the U.S. population by the first half of 2021," said Slaoui of Operation Warp Speed, in October. And that definitely will be the case. We will have 600 million to 700 million doses or more by May or June 2021."
Thanks to unprecedented government investments, an impressively coordinated scramble by several industries, and some fortuitous technological advances, Slaoui's boast seems credible. Since April, Stacy Springs and Donovan Guttieres at M.I.T.'s Center for Biomedical Innovation have been collecting data about each step of the supply, production, and distribution chains for COVID-19 vaccines. They have built models to investigate dependencies and identify critical points where shortages could interrupt production.
So far, Springs says, they have seen companies and agencies cooperating to spot problems and fix them: A lot of the manufacturers are already moving to dual sourcing of materials and putting in other safety nets, so that they're not going to be in a position where they don't have what they need." Although governments have been competing with one another to some extent to preorder vaccine for their own people, there's a lot of goodwill and sharing going on within the industry," she says.
It is indeed encouraging to learn that the immense efforts being mounted now to vaccinate the world against COVID-19 are being undertaken in a cooperative spirit. Perhaps, after a year of divisiveness and social isolation, the realization is dawning that we're all in this together.