Environmental and Permitting Considerations for Decarbonizing with Hydrogen

Many experts believe hydrogen will play a key role in the effort to decarbonize the world's energy supply. However, current hydrogen production methods release a lot of carbon dioxide, and carbon capture technology comes with its own complications and added costs. Burning hydrogen in gas turbines also raises NOx emission concerns, so there are still obstacles to overcome before hydrogen can fulfill its promise.
Hydrogen is the most abundant substance in the known universe. It has been studied intensely and used industrially-from agriculture to rocket fuel-for decades. It's the smallest, lightest element in the periodic table, and has both one of the highest gravimetric energy densities and lowest volumetric energy densities of gaseous fuels. In short, hydrogen is, scientifically, a well-understood element.
Despite this history and the global interest in hydrogen as a method to help decarbonize the power generation sector, the use of hydrogen as an electric power generation fuel source remains in its infancy. The notion of using hydrogen to decarbonize is multifaceted, but the motivation is clear-the combustion of pure hydrogen results in zero emissions of carbon dioxide (CO2). That said, there are a variety of environmental considerations associated with both hydrogen production and hydrogen combustion for power generation that necessitate further exploration.
Hydrogen ProductionHydrogen can be produced by several methods, categorized by different colors. In the power generation industry, the most applicable production methods are:
- Green Hydrogen. Electrolysis of water using renewable electricity.
- Grey Hydrogen. Steam methane reforming (SMR).
- Blue Hydrogen. SMR with carbon capture and sequestration (CCS).
Steam Methane Reforming with Carbon Capture and Sequestration. Currently, the most common method for producing hydrogen uses natural gas (methane) as a primary feedstock for SMR. Until recently, few have paid attention to CO2 emissions from SMR; however, with the increasing importance placed on decarbonization in the power generation sector, SMR is generally only viewed as a legitimate decarbonization technique when employed with CCS.
Efficient SMR processes can produce hydrogen (H2) from methane (CH4) and water (H2O) while emitting CO2 at a rate of about 90 pounds per million Btu (lb/MMBtu) of fuel produced. However, additional CO2 emissions can be attributed to the SMR process.
Significant energy input is required for the SMR process (heat and steam). When including CO2 emitted from their generation, the effective CO2 emission rate for electric production is about 160 lb/MMBtu. In comparison, the CO2 emission factor from direct combustion of natural gas in power generating equipment is far less, approximately 117 lb/MMBtu of fuel consumed.
Based on these approximations, the effective CO2 emissions from the entire SMR process would need to be reduced by about 30% for this hydrogen production method (and, ultimately, hydrogen-fired power generation) to begin decarbonizing. Adding CCS can help, but adds other complications, including the increased energy requirement to operate a CCS installation and the multiple exhaust streams (SMR process and energy generation) requiring treatment. Ultimately, with the current limitations of CCS technology, blue hydrogen has only a slightly smaller carbon footprint than grey hydrogen, with significantly higher capital and operating costs.
Electrolysis of Water. Hydrogen produced by the electrolysis of water, using electricity from renewable energy sources, has low CO2 emissions directly attributed to the process. Advantages of tying renewable energy sources to this hydrogen production process are twofold:
- A near-zero CO2-emitting source of energy is used in the production of hydrogen.
- The storage of hydrogen for later use in power production helps balance long-term, excess renewable power produced during lower demand periods and insufficient renewable power produced during higher demand periods.
This storage of excess renewable energy has been a challenge in the industry and is only partially addressed by using batteries for shorter periods of storage and smaller discharge capacities. Figure 1 highlights some of the more well-known energy storage technologies, showing hydrogen's potential to fill a large gap at the high end of both axes.
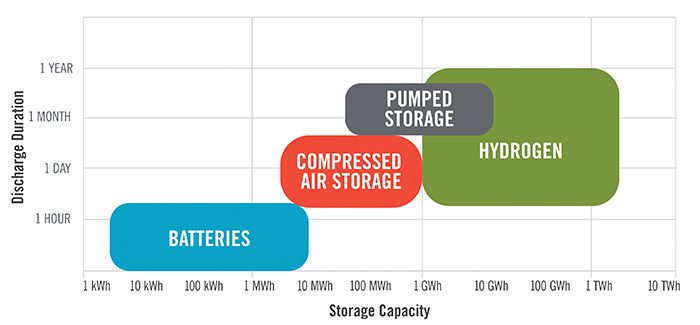 |
1. This graph shows the storage capacity (horizontal axis) and discharge duration (vertical axis) of four common energy storage technologies. Courtesy: POWER Engineers |
Hydrogen's potential for high-capacity, extended-duration energy storage is perhaps its biggest selling point as we move toward decarbonization with renewables as the predominantly growing asset class. But what are the environmental impacts of the process? Let's look at air quality and water resources.
Because there are no significant air emission sources at an electrolysis plant, air permitting issues are not expected to be significant. However, there are dispersion-related requirements, particularly those associated with venting hydrogen into the atmosphere during startup, shutdown, or malfunction events.
These vents are typically designed as an elevated flare (unlit) with the requisite radiative heat modeling that ensures a safe zone around the vent. In addition, appropriate light-gas dispersion modeling studies ensure that the released hydrogen plume is safely dispersed.
Electrolysis converts water into hydrogen and oxygen, and the production of one pound of hydrogen requires at least nine pounds of water. For example, burning 100% hydrogen in a 34-MW simple-cycle combustion turbine requires 4,675 pounds of hydrogen per hour. Assuming the hydrogen is generated at the same rate that the combustion turbine consumes it, 5,045 gallons of water per hour would be required to produce the hydrogen.
To place this in context, compare it to the water usage of golf course irrigation. According to the U.S. Golf Association, a typical golf course in the northeastern U.S. uses an annual average of 2,400 gallons of water per hour, while a typical golf course in the southwestern U.S. uses an annual average of 12,000 gallons of water per hour.
Hydrogen Combustion for Power GenerationAs fossil-fueled power generating plants are retired in lieu of renewable energy production, having the ability to deliver high-capacity, extended-duration decarbonized power becomes increasingly important. The shift of baseload generation to renewables makes hydrogen an increasingly attractive option for times when power demand is high and renewables are not available.
To generate utility-scale power during peak demand, hydrogen-fired combustion turbines are the technology of choice. Currently, hydrogen must be blended with natural gas. Most combustion turbine manufacturers claim that existing fleets can burn a fuel blend with up to 25% hydrogen by volume without burner modification. As technology quickly advances, the introduction of turbines that burn 100% hydrogen is coming soon.
Permitting Considerations and Design ImplicationsThere are, however, environmental issues associated with hydrogen combustion for power generation. A common assumption is that hydrogen is a clean-burning fuel, free from emissions of harmful air pollutants. While hydrogen as a fuel does not emit greenhouse gases, a combustion unit uses ambient air to supply the oxygen in the fuel combustion process. This air contains nitrogen that, in the presence of heat and oxygen, forms oxides of nitrogen (NOx). NOx emissions are highly regulated air pollutants by federal, state, and local environmental agencies.
NOx emissions increase when comparing hydrogen combustion to natural gas combustion. Hydrogen is lighter, burns hotter, and carries one-third the heating value on a volumetric basis compared to natural gas. Because it burns hotter, NOx emissions are higher. This increase in NOx emissions will likely trigger additional air permitting complications for existing units, and challenges in the design and operation of new units.
For existing units, adding the capability to burn 25% hydrogen to an air permit could be considered a change in the method of operation, triggering additional, more-extensive air permitting requirements. If there is no increase in NOx emissions, the air permitting complications can be minimized. If there is an increase in NOx emissions, this could trigger Prevention of Significant Deterioration (PSD) permitting requirements. These requirements can include air dispersion modeling to demonstrate compliance with air quality standards and a Best Available Control Technology (BACT) analysis. Increased NOx emissions for older turbines, which are historically subject to New Source Performance Standards (NSPS) Subpart GG, could mean they are now subject to the more restrictive NOx emission monitoring and limits of NSPS Subpart KKKK.
If a new combustion turbine is installed and will eventually burn 100% hydrogen, the future increase in NOx emissions should be considered early in the design process. The selective catalytic reduction system (SCR) should be sufficiently sized so that as the hydrogen content in the fuel blend increases, the corresponding increase in NOx emissions is minimized. Overdesigning the SCR up front will save permitting headaches and SCR redesigns in the future.
Furthermore, considering the increased hydrogen content will be important when conducting the air pollutant dispersion modeling analyses to ensure a sufficient compliance margin for future NOx emission increases. This might lead to an increased stack height during the initial design of the unit.
The purpose of burning hydrogen is to reduce CO2 emissions. With the limitations of today's technology, however, hydrogen is blended with natural gas. The relationship between fuel-blend hydrogen content and CO2 emission decreases is not linear. Figure 2 shows CO2 emissions based on the amount of hydrogen blended with natural gas on a volumetric basis.
 |
2. This graph depicts the relationship between the percent hydrogen in fuel blend (horizontal axis) and the CO2emission factor (left-hand vertical axis) and corresponding reduction in CO2emissions compared to natural gas firing (right-hand vertical axis). Courtesy: POWER Engineers |
As illustrated in Figure 2, when burning a 25% hydrogen blend, the CO2 emission reduction is about 10%. This reduction is caused by the lower gas density of hydrogen and corresponding low volumetric heating content compared to natural gas (that is, at 25% of the volumetric fuel blend, the hydrogen only contributes to about 10% of the fuel blend heating value).
A 10% CO2 emission reduction may not seem large enough to justify modifications to an existing combustion turbine, but it is currently the only achievable way to get such a reduction from an existing combustion turbine without adding CCS. Another prominent CO2 emission reduction method is heat rate improvement, but it is not realistic to get a 10% improvement in heat rate from any well-maintained utility combustion turbine.
Looking AheadHydrogen combustion is one of the few technologies that lowers CO2 emissions from familiar combustion sources. Consequently, as hydrogen production, storage, and combustion technologies mature, the role of hydrogen in decarbonization will increase.
As with any developing power generation technology, considering the big picture when planning for the future is essential to long-term success. The environmental and permitting implications of hydrogen, such as CO2 emissions from hydrogen production and NOx emission increases from hydrogen combustion, must be evaluated and understood for a project to be successful.
-Tom Rolfson, PEandSteven Bablerare air quality engineers for POWER Engineers, an engineering and environmental consulting firm.
The post Environmental and Permitting Considerations for Decarbonizing with Hydrogen appeared first on POWER Magazine.