Molecular monitor
Oncologists often turn to chemotherapy, an aggressive treatment that often relies on trial and error. It can be difficult to tell how many cancer cells chemotherapy has destroyed-let alone why different tumors may respond to the same treatment in different ways.
Hadley Sikes, the Esther and Harold E. Edgerton Associate Professor of Chemical Engineering at MIT and a principal investigator of the Antimicrobial Resistance Interdisciplinary Research Group at the Singapore-MIT Alliance for Research and Technology, has found a better way to monitor that progress. The key is something that has always fascinated her: so-called redox chemistry-reactions in which a molecule gains electrons (known as reduction) or loses them (known as oxidation).
In the body, unchecked oxidation destroys cells' normal function, and cancer is one of the potential consequences. The good news is that this excessive oxidation leaves a chemical signature. With the right tools, it can be detected.
In 2014, Sikes began wondering if this chemistry could form the basis for a visual representation of chemo's effects. What if scientists could monitor the oxidation happening in tumors-and see exactly where the treatment is and isn't working? With the aid of fluorescent proteins gleaned from jellyfish genes, she and her team were able to apply their knowledge of the redox chemistry to create innovative biosensors that track oxidation levels to see if tumors are expanding or shrinking.
Middle school biochemistFrom a young age, Sikes looked at the world with an insatiable curiosity about how things worked. She collected and observed everything from rocks to snakes. I drove my elementary school teachers crazy," she says.
In middle school, she was already designing experiments to measure the chemical reactions in nature, including a toxicology study of caffeine's effects on sea urchins. She'd hoped to persuade her father-a scientist himself-to moderate his coffee habit. While the experiment was unsuccessful in that regard, it planted a seed for something greater. Sikes was realizing how chemistry research could promote good health and benefit society.
Although her undergraduate studies at Tulane focused on physical chemistry, Sikes eventually circled back to her early biochemical research. At Stanford, where she earned her PhD, she began studying redox mechanisms, particularly how certain oxidizing agents pull electrons from other molecules. And she became interested in oxidative stress, which occurs when free radicals in the body-highly reactive molecules missing one or more electrons that readily oxidize other substances-overwhelm the antioxidants that cells normally produce to neutralize them. This can cause a variety of health problems.
In particular, cancer is characterized by higher-than-usual levels of free radicals called reactive oxygen species (ROS). In normal metabolic activity, ROS molecules promote cell regeneration and gene expression. But elevated ROS production can harm normal cells and facilitate tumor growth.
As a biochemist, Sikes was fascinated by the prospect of sensing and manipulating these changes, which doctors have struggled to measure accurately in cancer cells. To see what was happening inside tumors, she needed to see when cells were oxidized; she turned to fluorescent proteins that emit light at different wavelengths. To detect those redox reactions, we use chemistry that's triggered by light," says Sikes.
It was only a short step to translate that into therapeutic potential. If doctors can understand the actual redox activity underlying a tumor, they can better predict how chemotherapy will arrest that activity-and allow normal cells to regain control.
Otherwise, they'll continue shooting in the dark. Sikes had a vision of illuminating their quest-literally.
Sensors at workUsing her sensors, researchers could potentially measure when, where, and how much the tumors are experiencing oxidation-simply by lighting them up. The fluorescent sensors could also shed light on various therapeutics' mechanism of action, thereby helping doctors select the best ones for each patient.
Since 2018, Sikes's team has been collaborating with Tufts pathologist Arthur Tischler to use their biosensors for insight into the redox chemistry behind various cancers. In a paper published in 2020, they explored the pathology of tumors deficient in succinate dehydrogenase (SDH), a crucial metabolic enzyme and an inhibitor of ROS production. Low levels of SDH have been linked to cancers that are both rare and difficult to treat.
By reengineering biochemical processes, she can measure the distinctive chemistry behind antibody production, tumor development, and virtually all aspects of human disease.
Using the same biosensors, Sikes and her team became the first to focus on chemotherapies that induce a single oxidizing agent: hydrogen peroxide. In a paper published in Cell Chemical Biology, they outline how they created a sensor specifically designed to detect increased hydrogen peroxide concentrations, which can selectively kill cancer cells. The team examined 600 molecules as potential therapeutics, identifying four that boosted hydrogen peroxide in the tumor samples.
The team's achievement will facilitate clinical trials of new pharmaceuticals. The next step, ideally, is to use those fluorescent sensors to evaluate the effects of those therapeutics in patient-derived tumors.
Rapid-detection diagnosticsSikes realized that her technique could also detect pathogens-including SARS-CoV-2, the novel coronavirus that causes covid-19.
To make such a detector, Sikes needed antibody proteins that would react with the distinctive proteins of the virus. But those reactive proteins didn't exist. So she decided to create them.
In her postdoc research, Sikes had worked with Caltech chemical engineer and 2018 Nobel laureate Frances Arnold, a pioneer in creating novel proteins with desirable properties.
Sikes's lab now engineers proteins that lock onto the distinctive folds in the proteins characteristic of various pathogens. The engineered proteins emit different wavelengths depending on how they bond with the virus's or bacterium's material.
On the basis of this innovative technology, Sikes has developed rapid diagnostic tests incorporating a set of reagents that find one species and exclude the others, so health professionals can more quickly and accurately diagnose infectious diseases. Her lab focuses on engineering reagents that can identify coronaviruses, respiratory syncytial virus (RSV), and other causes of respiratory disease; bacteria that affect food safety (particularly Listeria and E. coli); and parasitic eukaryotes such as Plasmodium, which causes malaria.
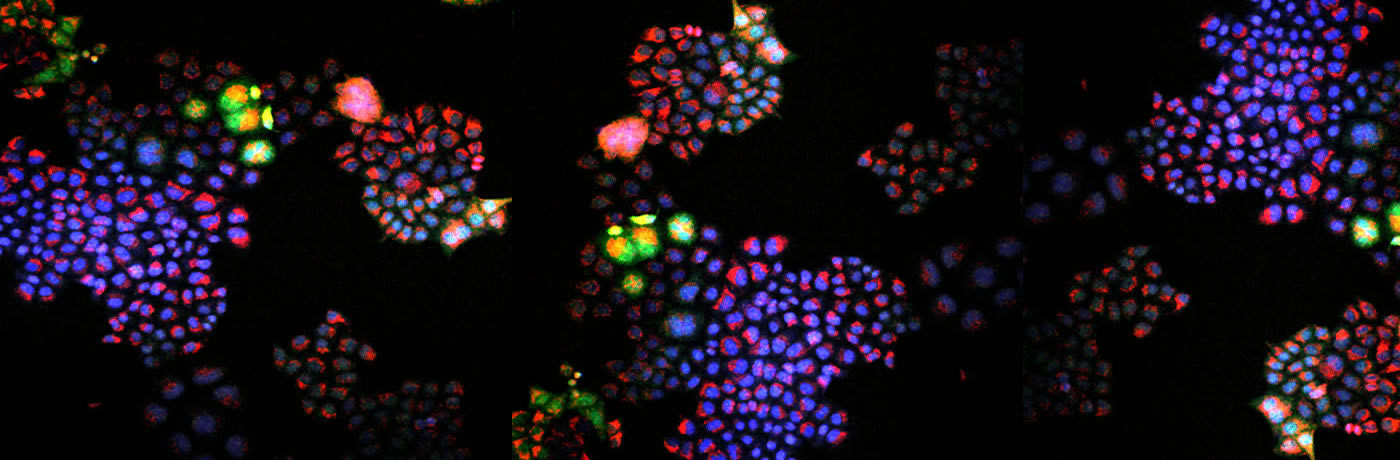 Fluorescence microscopyimage of a tumor sample where elevated levelsof hydrogen peroxide have been discovered.COURTESY OF THE RESEARCHERS
Fluorescence microscopyimage of a tumor sample where elevated levelsof hydrogen peroxide have been discovered.COURTESY OF THE RESEARCHERSSikes's students and postdocs at her Singapore lab are now developing tests that assess immunity against different covid-19 variants as part of a fast-tracked research project. As in her other studies, specially engineered proteins will react uniquely with each person's repertoire of antibodies-allowing the team to better understand the extent and durability of immunity to covid on an individual level.
Sikes's effort to save lives with emerging biosensor technology is just part of her mission to use chemistry research for society's benefit. She accepted her position at MIT in 2009 largely because of its reputation for research that could be applied to solve social problems. And to advance that mission still further, she cherishes her opportunities to mentor aspiring scientists.
Every summer, MIT accepts budding researchers from historically underrepresented areas and schools. Last summer, Sikes mentored students from Spelman College, Morehouse College, and the University of Puerto Rico-Mayaguez. The program offers hands-on opportunities to do research and build connections with the Institute's network of scientists. As part of an MIT exchange program, Sikes also mentors undergraduates at Imperial College London.
To Sikes, this is the epitome of what science education should be. I learn probably as much from them as they learn from me," she says. I really view it as a collaboration. I've been doing this for 20 years now ... but all these students and postdocs come with their own backgrounds and experiences and ways of looking at things. Often, they have ideas or hypotheses that wouldn't have occurred to me."
Redox to the rescueThe mysteries that Sikes has been chasing since childhood have all come down to measurement: What invisible reactions drive surface phenomena?
Today, by reengineering biochemical processes, she can measure the distinctive chemistry behind antibody production, tumor development, and virtually all aspects of human disease. In the next few years, she hopes to finalize the biosensor proteins and get them to market, empowering other researchers to improve patient outcomes and mitigate the next pandemic.
That's not to say Sikes's lifelong curiosity has been sated. There are always further questions to be asked. I hope 10 years from now we'll be doing something totally different that I can't even imagine right now," she says.