Facebook is bombarding cancer patients with ads for unproven treatments
The ad reads like an offer of salvation: Cancer kills many people. But there is hope in Apatone, a proprietary vitamin C-based mixture, that is KILLING cancer." The substance, an unproven treatment that is not approved by the FDA, is not available in the United States. If you want Apatone, the ad suggests, you need to travel to a clinic in Mexico.
If you're on Facebook or Instagram and Meta has determined you may be interested in cancer treatments, it's possible you've seen this ad, or one of the 20 or so others recently running from the CHIPSA hospital in Mexico near the US border, all of which are publicly listed in Meta's Ad Library. They are part of a pattern on Facebook of ads that make misleading or false health claims, targeted at cancer patients.
 One CHIPSA ad that is still live, according to Meta's Ad Library. (MIT Technology Review)
One CHIPSA ad that is still live, according to Meta's Ad Library. (MIT Technology Review) Evidence from Facebook and Instagram users, medical researchers, and its own Ad Library suggests that Meta is rife with ads containing sensational health claims, which the company directly profits from. The misleading ads may remain unchallenged for months and even years. Some of the ads reviewed by MIT Technology Review promoted treatments that have been proved to cause acute physical harm in some cases. Other ads pointed users toward highly expensive treatments with dubious outcomes.
CHIPSA, which stands for Centro Hospitalario Internacional del Pacifico, S.A, was founded in 1979 and refers to itself as a community hospital offering integrative treatments for cancer. On Facebook, the facility describes itself as being at the cutting edge" of cancer research. But the hospital's foundational diet-based therapy, called the Gerson Protocol, is all nonsense," says David Gorski, a surgical oncologist at Wayne State University in Michigan and the managing editor of the website Science-Based Medicine. Developed by a German doctor in the 1920s to treat migraines, the regimen consists of a special diet and frequent detox" procedures. It has been discredited for decades in the medical community.
CHIPSA did not respond to repeated requests via phone and email for comment.
MIT Technology Review alerted Meta to five CHIPSA ads, along with three ads from another international clinic called Verita Life. In response, Meta spokesperson Mark Ranneberger said that it had removed several of the ads for violating our misleading claims policy, which prohibits claims of cures for incurable diseases."
When asked for the specifics of the ads removed, Ranneberger said that two were rejected: the one claiming that Apatone was killing" cancer and another that mentioned growing distrust" of the US health-care system while advertising exclusive cancer treatments. Another ad using identical text to that second one but a different image remains active. On Monday, after the publication of this article, Meta noted that it had removed three additional ads using the same language.
Us cancer patients and survivors, we are just bombarded with all these kinds of alternative things all the time," says Nikhil Autar, a medical student in Australia who has acute myeloid leukemia. Autar started seeing ads for cancer treatment centers on Facebook in 2019-just as Facebook and other platforms began rolling out new policies designed to limit the reach of health misinformation.
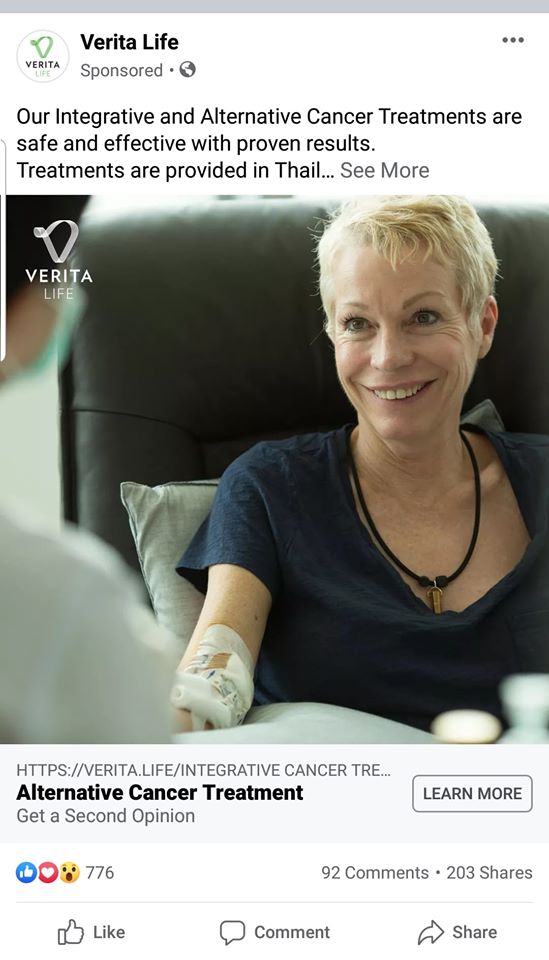 A screenshot of an ad captured by Nikhil Autar on August 12, 2020.
A screenshot of an ad captured by Nikhil Autar on August 12, 2020. Facebook has drastically stepped up its efforts to stop the spread of sensational and false health claims over the past few years. After a series of local measles outbreaks in the US in 2019, it announced it would start treating misleading health claims like spam, reducing their reach on news feeds and limiting the visibility of private Facebook groups promoting dubious treatments. When the covid-19 pandemic began, the company rolled out more comprehensive efforts to remove or limit such claims as conspiracies about the virus, masks, and vaccines spread on its platform.
These attempts to combat pitches for miracle cures and dubious medical advice have been a step in the right direction, says Rachel Moran, a postdoctoral fellow at the Center for an Informed Public at the University of Washington. However, many such ads continue to slip through.
One from Verita Life, in Bangkok, Thailand, targeted Australians like Autar, falsely claiming that a hypothermia treatment offered there would destroy cancer cells." When Autar took a screenshot of the ad in his news feed in August of 2020, it had more than a thousand likes and 600 shares.
Autar reported the ads he saw to Facebook using its in-platform systems, but they remained up. At one point, he says, he used a Silicon Valley connection to try to flag the ads directly to Facebook management. He stopped seeing the clinic's ads in the Ad Library and on his own feed after that, but they returned a few months later.
Both CHIPSA and Verita Life had several ads running on Facebook and Instagram before MIT Technology Review inquired about them, according to the Ad Library. Verita Life was able to place an ad as recently as June 18, 2022, promoting the testimonial of a patient with prostate cancer. MIT Technology Review flagged that ad, along with two others promoting the same testimonial. All three remain active.
 One of CHIPSA's ads, removed after we flagged it to Meta (MIT Technology Review)
One of CHIPSA's ads, removed after we flagged it to Meta (MIT Technology Review) Meta reviews new ads through a largely automated process before they go live. The company noted that ads and posts from CHIPSA's Facebook page and Instagram account are eligible to be flagged and fact-checked by third-party fact checkers. If a company repeatedly violates its policies, Meta says, it will temporarily suspend the company's ability to place ads.
While Meta has rules pertaining to, for instance, misleading claims in ads, all Facebook and Instagram ads must also follow Meta's community guidelines. The guidelines ban content promoting or advocating for harmful miracle cures for health issues" when those claims both contribute to serious injury or death and have no legitimate health use.
Those rules, even when swiftly enforced, can leave a lot of gray area for sensational claims, Gorski says, because a lot of quackery could have a legitimate health use." For instance, he says, vitamin C obviously has legitimate health uses; it just doesn't cure cancer."
So what about Apatone, the treatment advertised by CHIPSA? Pre-clinical research indicates some anti-cancer effect, but it has not been demonstrated to be more beneficial than standard treatments we are using currently in humans," says Skyler Johnson, a cancer researcher who studies misinformation at the University of Utah.
The danger is not simply that the treatments are unproven or ineffective. Some alternative cancer treatments advertised on the platform can cause physical harm. Coley's toxins, a treatment developed in the late 19th century and offered at CHIPSA, comes with risks including infection, site reactions, anaphylaxis, and in severe cases shock, says Johnson.
Unproven treatments can also interact poorly with conventional treatments like chemotherapy should a patient decide to pursue alternative care on their own. Moreover, simply delaying the start of proven therapies by detouring into unproven ones can allow the cancer to advance, complicating and diminishing the effectiveness of further treatment.
Johnson's research has demonstrated worse survival rates for patients who seek unproven cancer treatments at first. In a 2017 study, he found that after about five years, patients with breast cancer who delayed conventional treatment in favor of alternative medicine were more than five times more likely to die.
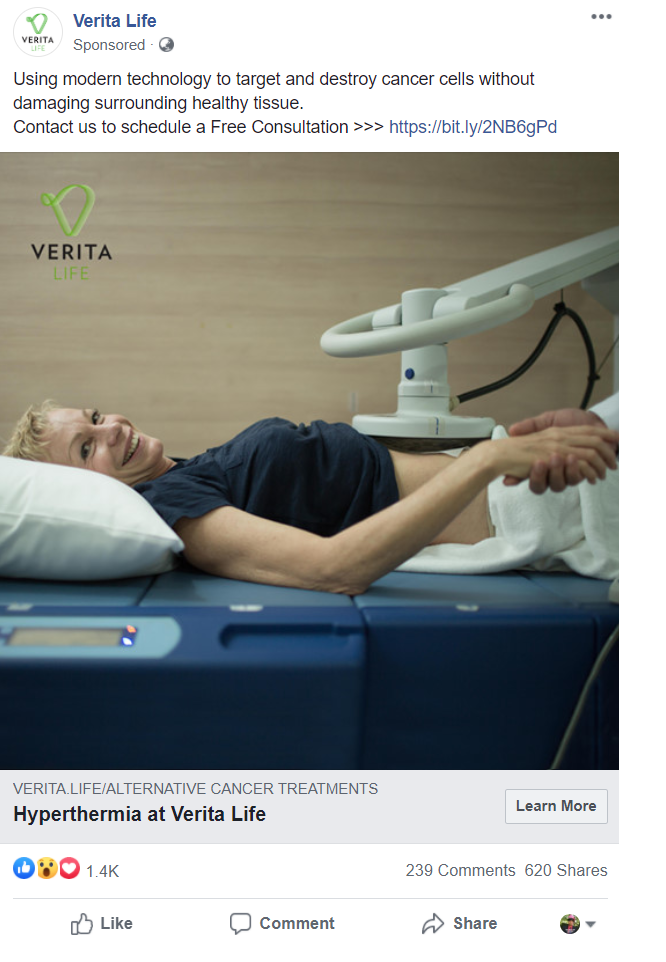 An ad containing a false claim about hyperthermia as a cancer treatment, captured by Autar in August 2020.
An ad containing a false claim about hyperthermia as a cancer treatment, captured by Autar in August 2020.There's the financial burden, too-because clinics like CHIPSA aren't generally covered by insurance, patients often have to raise money to afford their treatments. One recent GoFundMe campaign for a cancer patient seeking treatment at CHIPSA included a screenshot of a bill for the base amount" he'd have to pay. It was $36,500 for three weeks of inpatient care in Mexico. That cost would increase once the facility decided on a treatment plan.
CHIPSA has spent about $5,000 since mid-2018 advertising on Meta about social issues, politics, or elections, according to information available in the Ad Library before Meta removed two of its ads. CHIPSA did not respond to requests for details on its ad spending or the cost of the treatments it offers.
Gorski is blunt about his view on whether Facebook will effectively address cancer misinformation: The only real way to combat such misinformation on Facebook would require an army of fact checkers that Facebook is never going to pay for, given its past record even on covid-19 misinformation and dangerous political conspiracy theories."
And as the University of Washington's Moran points out, misinformation like this rarely stays confined to the platform where it's originally posted. While Facebook plays a key role in getting sensational claims about dubious cancer treatments in front of desperate patients, the groups and ads carrying those claims often link to other sites and networks that reinforce them.
Johnson, using data from 2017 to 2019, has observed that articles and videos containing myths about cancer treatment often receive more social media engagement than those from safe" sources. And although it's tricky to say for sure, his and other research in this area suggests that as many as one in three online articles or videos posted online about cancer may contain harmful misinformation.
Especially when you are experiencing a medical crisis, you are looking at an incredible amount of information," Moran says. It seems good to you that you are doing your research, you're going from one site to the next. But they all belong to the same ecosystem."
This post has been updated with additional information from Meta
If you or a loved one has been treated for cancer, we'd like to talk to you for future stories about sensational health claims on social media. If you are a member of support groups for cancer patients or their loved ones, or have experience with clinics like the ones mentioned in this story, please be in touch: abby.ohlheiser@technologyreview.com