The SpaceX Nine Raptor Mars Rocket
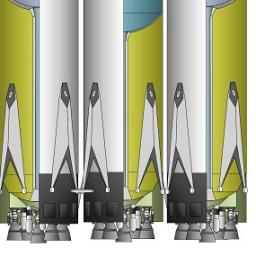 The ever-improving Raptor rocket engine from SpaceX has mutated to a 1Mlbf (4,500kN) gas-gas (full flow) liquid methane and oxygen engine. With nine such engines in an arrangement that could dwarf even the Saturn V, can Musk and his team finally shoot for Mars?
The ever-improving Raptor rocket engine from SpaceX has mutated to a 1Mlbf (4,500kN) gas-gas (full flow) liquid methane and oxygen engine. With nine such engines in an arrangement that could dwarf even the Saturn V, can Musk and his team finally shoot for Mars?