Some aging reversal in appearance, liver, muscle and other functions with cell penetrating peptides in very old mice
by noreply@blogger.com (brian wang) from NextBigFuture.com on (#2GVET)
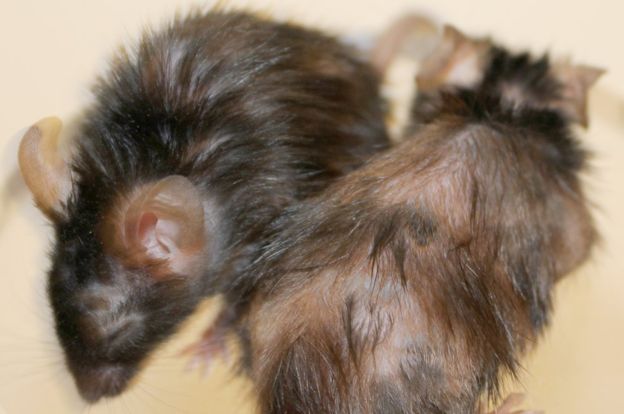
Researchers have rejuvenated old mice to restore their stamina, coat of fur and even some organ function. The team at Erasmus University Medical Center, in the Netherlands, are planning human trials for what they hope is a treatment for old age. A UK scientist said the findings were "impossible to dismiss", but that unanswered questions remained. The approach works by flushing out retired or "senescent" cells in the body that have stopped dividing.
They tested it on mice that were just old (the equivalent of 90 in mouse years), those genetically programmed to age very rapidly and those aged by chemotherapy.
The findings, published in the journal Cell, showed liver function was easily restored and the animals doubled the distance they would run in a wheel.
Dr de Keizer said: "We weren't planning to look at their hair, but it was too obvious to miss.
The drug was given three times a week and the experiments have been taking place for nearly a year.
With life expectancy projected to increase in the foreseeable future, it is important to develop strategies to extend and restore healthspan. Cell-penetrating peptides (CPPs) are relatively understudied in aging research. Further analysis of their use is warranted as they serve several major advantages. Counter to broad-range inhibitors, CPPs can in theory target any surface-exposed stretch of amino acids to block specific protein-protein interactions and, in doing so, they can selectively modulate very specific downstream signaling events (discussed in de Keizer (2017))). Other compounds, classified as senolytics, have been described to influence senescent cell viability. As a CPP, FOXO4-DRI differs from these by being designed around a specific amino acid sequence in a molecular target only mildly expressed in most normal tissues (see e.g., Figures S2J and S2K). Though a more thorough analysis is required, at least as far as tested here FOXO4-DRI appears to be well tolerated, which is an absolutely critical milestone to pass when aiming to treat relatively healthy aged individuals.
FOXO4-DRI effectively disrupts the p53-FOXO4 interaction, but the importance of the FOXO4 protein itself is more complicated in DNA damage and senescence. As FOXO4-DRI causes nuclear exclusion of active p53, the levels of p21Cip1 decline. However, the loss of p21Cip1 alone is insufficient to induce apoptosis and was actually shown to induce a senescence-escape instead (Brown et al., 1997). Rather, the exclusion of p53 itself has been reported to induce apoptosis directly when relocated to mitochondria (Mihara et al., 2003), thereby explaining the FOXO4-DRI effects. FOXO4 shRNAs induce apoptosis in senescent IMR90, arguing that full FOXO4 inhibition might also be of use against senescence. True as this may be, chronic FOXO4 reduction is not advisable as FOXOs play a role in DNA-damage repair and Foxo4a'/a' mice are susceptible to acute damage (Zhou et al., 2009). In contrast to loss of FOXO4, FOXO4-DRI does not sensitize healthy cells to acute DNA damage (Figure 4G). Thus, while permanent FOXO4 inhibition is inapplicable, the fact that as a CPP it can block a specific protein-protein interaction makes FOXO4-DRI selective and thereby well tolerated and effective.
Based on these positive effects, it is now possible to envision a point on the horizon where the disease indications are identified that could benefit most from FOXO4-DRI therapy. High SASP-secreting cells are likely to play a much larger role in disease development than more sterile senescent cells. Through SASP, senescent cells may permanently confer a state of stemness in neighboring cells and thereby impair tissue function and renewal, an effect that we recently described in the senescence-stem lock model for aging (de Keizer, 2017). FOXO4-DRI has a strong preference for targeting high-SASP subpopulations of senescent cells, but it is unclear what causes heterogeneity in the SASP. It will be a major achievement to unravel those mechanisms and to steer these such that therapeutic targeting is most beneficial. In that sense, identification of senescence-driven pathologies that rely on SASP may help in optimizing candidates for therapy. XpdTTD/TTD is a pleiotropic model for aging that can be effectively used as a basis for such research. It is a well-established model for osteoarthritis, especially in cohorts of older age than we used here (52 weeks) (Botter et al., 2011) and for the unhealthy loss in muscle (sarcopenia) and fat mass.
Last, it is relevant to note that independent of aging and age-related diseases, FOXO4-DRI may be of use against the progression, stemness, and migration of malignant cancer. Given that SASP factors influence these (Campisi, 2013), it will be particularly interesting to determine whether FOXO4-DRI affects those p53-wt cancer cells that have adopted a more migratory and stem-like state due to reprogramming by chronic SASP exposure. In any case, the here reported beneficial effects of FOXO4-DRI provide a wide range of possibilities for studying the potential of therapeutic removal of senescence against diseases for which few options are available.
Fightaging points out we really have little idea as to how the life extension observed in mice lacking senescent cells will scale in humans. Near all methods of extending life in mice to date have been based on modestly slowing aging, changing the operation of metabolism to reduce the rate at which molecular damage accrues. Short-lived species like mice have a much greater response to this sort of thing than do humans, demonstrated when we compare the effects of calorie restriction and growth hormone receptor loss of function mutations. In mice these can extend life by as much as half again, but if that was the case in humans, we'd have certainly noticed by now. Clearing senescent cells is a completely different form of therapy, however, a type of damage repair carried out intermittently rather than an ongoing slowing of damage. I know of no such approach that has been tried in both mice and humans, and thus there is no basis for comparison.

Highlights
The accumulation of irreparable cellular damage restricts healthspan after acute stress or natural aging. Senescent cells are thought to impair tissue function, and their genetic clearance can delay features of aging. Identifying how senescent cells avoid apoptosis allows for the prospective design of anti-senescence compounds to address whether homeostasis can also be restored. Here, we identify FOXO4 as a pivot in senescent cell viability. We designed a FOXO4 peptide that perturbs the FOXO4 interaction with p53. In senescent cells, this selectively causes p53 nuclear exclusion and cell-intrinsic apoptosis. Under conditions where it was well tolerated in vivo, this FOXO4 peptide neutralized doxorubicin-induced chemotoxicity. Moreover, it restored fitness, fur density, and renal function in both fast aging XpdTTD/TTD and naturally aged mice. Thus, therapeutic targeting of senescent cells is feasible under conditions where loss of health has already occurred, and in doing so tissue homeostasis can effectively be restored.
Cell - Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging
Read more










They tested it on mice that were just old (the equivalent of 90 in mouse years), those genetically programmed to age very rapidly and those aged by chemotherapy.
The findings, published in the journal Cell, showed liver function was easily restored and the animals doubled the distance they would run in a wheel.
Dr de Keizer said: "We weren't planning to look at their hair, but it was too obvious to miss.
The drug was given three times a week and the experiments have been taking place for nearly a year.
With life expectancy projected to increase in the foreseeable future, it is important to develop strategies to extend and restore healthspan. Cell-penetrating peptides (CPPs) are relatively understudied in aging research. Further analysis of their use is warranted as they serve several major advantages. Counter to broad-range inhibitors, CPPs can in theory target any surface-exposed stretch of amino acids to block specific protein-protein interactions and, in doing so, they can selectively modulate very specific downstream signaling events (discussed in de Keizer (2017))). Other compounds, classified as senolytics, have been described to influence senescent cell viability. As a CPP, FOXO4-DRI differs from these by being designed around a specific amino acid sequence in a molecular target only mildly expressed in most normal tissues (see e.g., Figures S2J and S2K). Though a more thorough analysis is required, at least as far as tested here FOXO4-DRI appears to be well tolerated, which is an absolutely critical milestone to pass when aiming to treat relatively healthy aged individuals.
FOXO4-DRI effectively disrupts the p53-FOXO4 interaction, but the importance of the FOXO4 protein itself is more complicated in DNA damage and senescence. As FOXO4-DRI causes nuclear exclusion of active p53, the levels of p21Cip1 decline. However, the loss of p21Cip1 alone is insufficient to induce apoptosis and was actually shown to induce a senescence-escape instead (Brown et al., 1997). Rather, the exclusion of p53 itself has been reported to induce apoptosis directly when relocated to mitochondria (Mihara et al., 2003), thereby explaining the FOXO4-DRI effects. FOXO4 shRNAs induce apoptosis in senescent IMR90, arguing that full FOXO4 inhibition might also be of use against senescence. True as this may be, chronic FOXO4 reduction is not advisable as FOXOs play a role in DNA-damage repair and Foxo4a'/a' mice are susceptible to acute damage (Zhou et al., 2009). In contrast to loss of FOXO4, FOXO4-DRI does not sensitize healthy cells to acute DNA damage (Figure 4G). Thus, while permanent FOXO4 inhibition is inapplicable, the fact that as a CPP it can block a specific protein-protein interaction makes FOXO4-DRI selective and thereby well tolerated and effective.
Based on these positive effects, it is now possible to envision a point on the horizon where the disease indications are identified that could benefit most from FOXO4-DRI therapy. High SASP-secreting cells are likely to play a much larger role in disease development than more sterile senescent cells. Through SASP, senescent cells may permanently confer a state of stemness in neighboring cells and thereby impair tissue function and renewal, an effect that we recently described in the senescence-stem lock model for aging (de Keizer, 2017). FOXO4-DRI has a strong preference for targeting high-SASP subpopulations of senescent cells, but it is unclear what causes heterogeneity in the SASP. It will be a major achievement to unravel those mechanisms and to steer these such that therapeutic targeting is most beneficial. In that sense, identification of senescence-driven pathologies that rely on SASP may help in optimizing candidates for therapy. XpdTTD/TTD is a pleiotropic model for aging that can be effectively used as a basis for such research. It is a well-established model for osteoarthritis, especially in cohorts of older age than we used here (52 weeks) (Botter et al., 2011) and for the unhealthy loss in muscle (sarcopenia) and fat mass.
Last, it is relevant to note that independent of aging and age-related diseases, FOXO4-DRI may be of use against the progression, stemness, and migration of malignant cancer. Given that SASP factors influence these (Campisi, 2013), it will be particularly interesting to determine whether FOXO4-DRI affects those p53-wt cancer cells that have adopted a more migratory and stem-like state due to reprogramming by chronic SASP exposure. In any case, the here reported beneficial effects of FOXO4-DRI provide a wide range of possibilities for studying the potential of therapeutic removal of senescence against diseases for which few options are available.

Fightaging points out we really have little idea as to how the life extension observed in mice lacking senescent cells will scale in humans. Near all methods of extending life in mice to date have been based on modestly slowing aging, changing the operation of metabolism to reduce the rate at which molecular damage accrues. Short-lived species like mice have a much greater response to this sort of thing than do humans, demonstrated when we compare the effects of calorie restriction and growth hormone receptor loss of function mutations. In mice these can extend life by as much as half again, but if that was the case in humans, we'd have certainly noticed by now. Clearing senescent cells is a completely different form of therapy, however, a type of damage repair carried out intermittently rather than an ongoing slowing of damage. I know of no such approach that has been tried in both mice and humans, and thus there is no basis for comparison.

Highlights
- A modified FOXO4-p53 interfering peptide causes p53 nuclear exclusion in senescent cells
- This FOXO4 peptide induces targeted apoptosis of senescent cells (TASC)
- TASC neutralizes murine liver chemotoxicity from doxorubicin treatment
- TASC restores fitness, hair density, and renal function in fast and naturally aged mice
The accumulation of irreparable cellular damage restricts healthspan after acute stress or natural aging. Senescent cells are thought to impair tissue function, and their genetic clearance can delay features of aging. Identifying how senescent cells avoid apoptosis allows for the prospective design of anti-senescence compounds to address whether homeostasis can also be restored. Here, we identify FOXO4 as a pivot in senescent cell viability. We designed a FOXO4 peptide that perturbs the FOXO4 interaction with p53. In senescent cells, this selectively causes p53 nuclear exclusion and cell-intrinsic apoptosis. Under conditions where it was well tolerated in vivo, this FOXO4 peptide neutralized doxorubicin-induced chemotoxicity. Moreover, it restored fitness, fur density, and renal function in both fast aging XpdTTD/TTD and naturally aged mice. Thus, therapeutic targeting of senescent cells is feasible under conditions where loss of health has already occurred, and in doing so tissue homeostasis can effectively be restored.
Cell - Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging
Read more