Physics not “broken” after all? We’re close to resolving proton radius puzzle
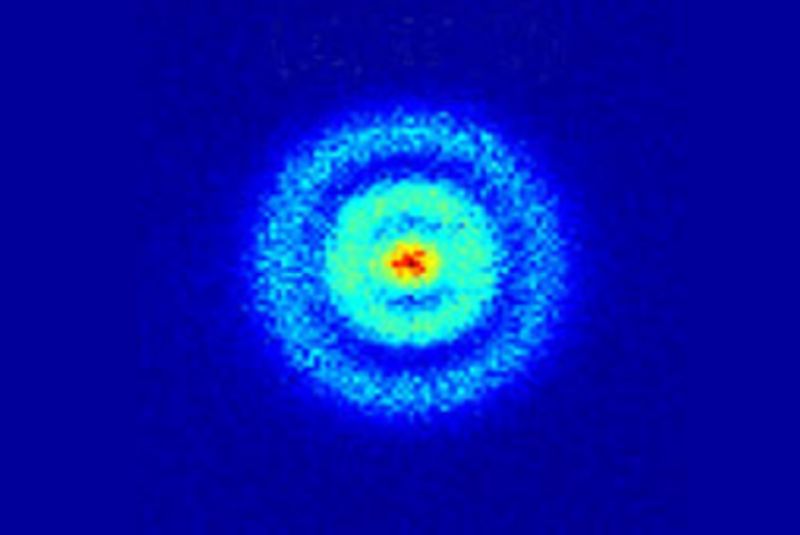
Enlarge / Image of a hydrogen atom's electron orbitals taken with a quantum microscope in 2013. Physicists have been trying to resolve conflicting experimental results, using hydrogen atoms, on the proton's radius for nearly a decade. (credit: APS/Alan Stonebraker)
Physicists at York University in Toronto have spent the last eight years meticulously conducting a sensitive experiment to measure the charge radius of the proton in hopes of resolving conflicting values obtained by several similar experiments performed over the last decade. That conundrum has been dubbed the "proton radius puzzle." The new results, published in a new paper in Science, confirm a 2010 finding that the proton is significantly smaller than scientists previously believed.
Most popularizations discussing the structure of the atom rely on the much-maligned Bohr model, in which electrons move around the nucleus in circular orbits. It's fine as a gateway drug to physics, so to speak, but quantum mechanics gives us a much more precise (albeit weirder) description. The electrons aren't really orbiting the nucleus; they are technically waves that take on particle-like properties when we do an experiment to determine their position. While orbiting an atom, they exist in a superposition of states, both particle and wave, with a wave function encompassing all the probabilities of its position at once. A measurement will collapse the wave function, giving us the electron's position. Make a series of such measurements and plot the various positions that result, and it will yield something akin to a fuzzy orbit-like pattern.
Quantum weirdness extends to the proton, too. Technically it's made of three charged quarks bound together by the strong nuclear force. But it's fuzzy, like a cloud. And how can we talk about the radius of a cloud? Physicists rely on the charge density to do so, akin to the density of water molecules in a cloud. The radius of the proton is the distance at which the charge density drops below a certain energy threshold. And it's possible to measure that radius by studying how the electron interacts with the proton, via either electron scattering experiments or by using electron or muon spectroscopy to look at the difference between atomic energy levels. (It's called the "Lamb shift," after Nobel laureate Wallis Lamb, who first measured the shift in 1947.) The combined fuzziness of the electron and proton means that the electron can be anywhere inside that region-including inside the proton.
Read 11 remaining paragraphs | Comments