A Chemistry Nobel we can use: Lithium-ion batteries
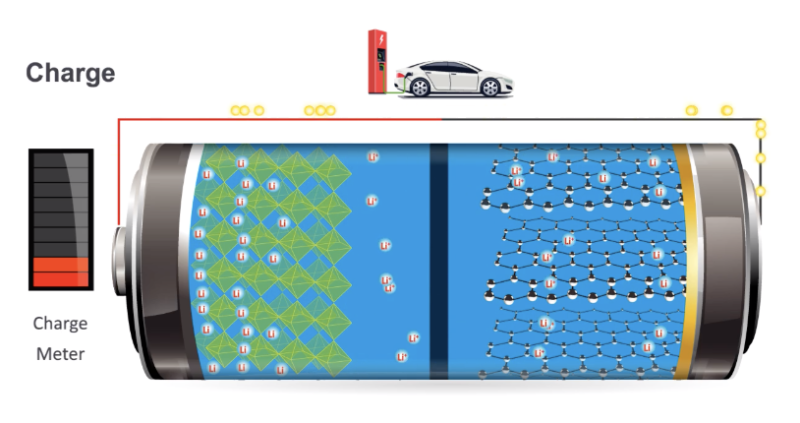
Enlarge / A diagram showing lithium ions moving between two different materials they can intercalate into. (credit: US DOE)
Lithium-ion batteries have become central to everything from the mobile-electronic revolution to electric vehicles, and they're poised to play a huge role in fostering the expansion of renewable power. It's safe to say that their development has made a discernible impact on the modern world. But aside from the fact that they involve lithium ions, the chemistry that enables these batteries to power so many things while balancing size, durability, and weight isn't widely understood.
Today's Nobel Prize in Chemistry provides a fantastic opportunity to correct that, as it honors three researchers who made key contributions to the development of the lithium-ion battery: Stanley Whittingham for developing the first intercalation-driven version, John Goodenough for developing the cathodes we use today, and Akira Yoshino for developing the anodes.
Less chemistry?The earliest batteries we developed were driven by chemical reactions. The lead-acid battery, for example, has two electrodes that undergo reactions with sulfuric acid in the electrolyte, liberating electrons to be used for other purposes. These reactions are the sorts of things you'd recognize from high school chemistry, with atoms swapping their partners and transferring electrons to end up in a different charge state. To an extent, the development of the lithium-ion battery was an attempt to get rid of all that.
Read 9 remaining paragraphs | Comments