Why Pfizer’s RSV vaccine success is a big deal, decades in the making
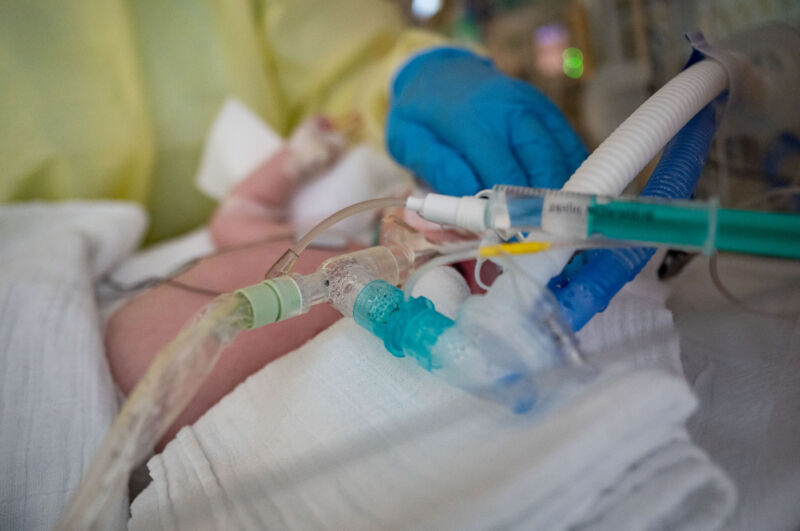
Enlarge / An intensive care nurse cares for a patient suffering from respiratory syncytial virus (RSV), who is being ventilated in the children's intensive care unit of the Olga Hospital of the Stuttgart Clinic in Germany. (credit: Getty | picture alliance)
As an unusually large and early seasonal surge of RSV cases inundate children's hospitals around the country, pharmaceutical giant Pfizer offered a glimmer of hope Tuesday in the form of top-line, phase three clinical trial results.
The company's experimental RSV vaccine-given to pregnant trial participants-was 82 percent effective at preventing severe RSV-related lower respiratory tract illness in the first three months of an infant's life. It was 69 percent effective over the first six months, Pfizer announced.
We are thrilled by these data as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth," Pfizer Chief Scientific Officer Annaliesa Anderson said in a statement.