No drug is safe: Drug developers decry Texas abortion pill ruling
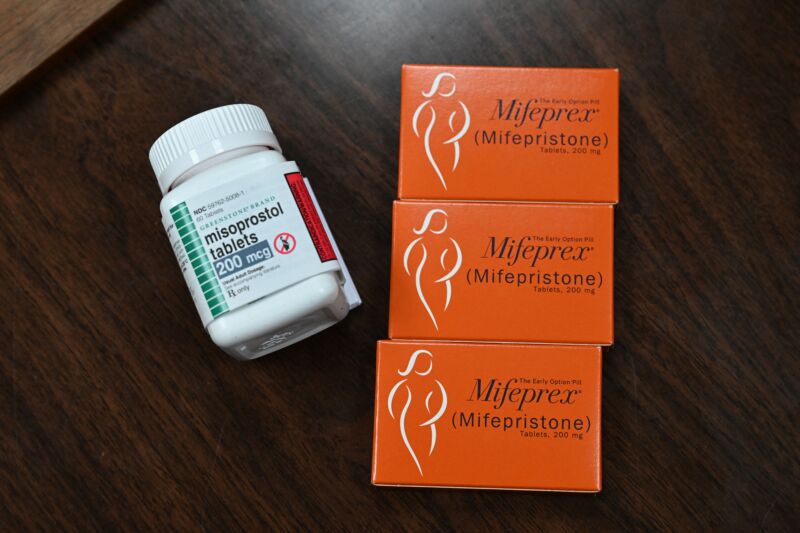
Enlarge / Mifepristone (Mifeprex) and Misoprostol, the two drugs used in a medication abortion, are seen at the Women's Reproductive Clinic, which provides legal medication abortion services, in Santa Teresa, New Mexico, on June 17, 2022. (credit: Getty | Robyn Beck)
A federal judge in Texas issued a ruling Friday to revoke the Food and Drug Administration's nearly 23-year-old approval of the safe and effective abortion and miscarriage medication, mifepristone. Although expected, the ruling throws into question the FDA's authority over all medicines and threatens to weaken the country's premier drug development pipeline, industry leaders and legal experts say.
In a public letter that circulated over the weekend, executives and leaders of the biotechnology and pharmaceutical industries condemned the ruling and called for its reversal along with "appropriate restitution" of the FDA's authority.
As of Monday afternoon, the letter had around 400 signatures and was accumulating more. Among them are big players in the industry, including Pfizer CEO Albert Bourla; Alisha Alaimo, president of Biogen; Christopher Tan, an executive for Merck & Co.; Imran Nasrullah, a vice president for Bayer Pharmaceuticals; and a senior clinical leader at Novartis, Nancy Lewis. But the vast majority are from smaller biotech companies, who stand to lose the most from downstream effects of the ruling, issued by District Judge Matthew Kacsmaryk.