COVID vaccines: FDA retires monovalent shots, offers spring boosters to some
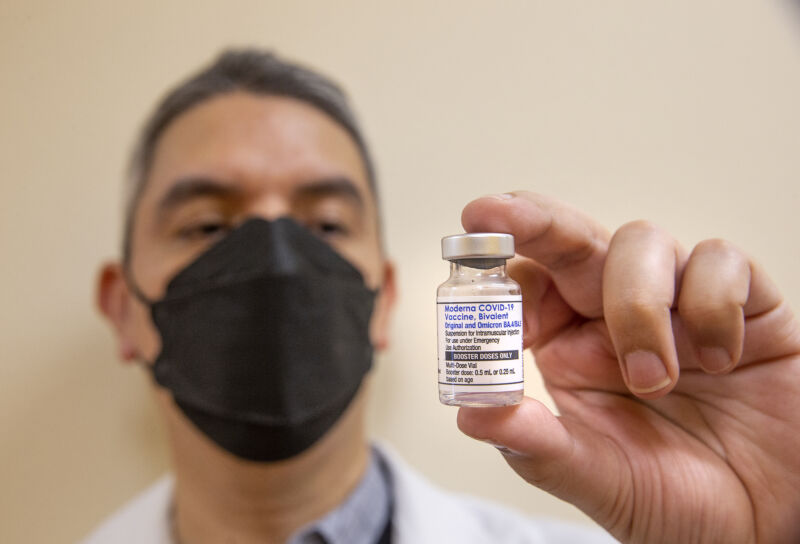
Enlarge / A pharmacist holds a bottle containing Moderna's bivalent COVID-19 vaccine. (credit: Getty | Mel Melcon)
The Food and Drug Administration on Tuesday altered its authorizations for mRNA-based COVID-19 vaccines, retiring the original monovalent versions entirely, streamlining immunizations for the unvaccinated, and offering spring bivalent boosters to those age 65 and older and people with certain immune-compromising conditions.
The changes will need a sign-off from Rochelle Walensky, director of the Centers for Disease Control and Prevention, before they go into effect. The agency will convene its advisory panel of vaccine experts Wednesday to discuss the changes. Walensky is likely to sign off soon after.
With the changes, the only mRNA-based COVID-19 vaccines authorized and in use in the country will be the bivalent formulations from Moderna and Pfizer-BioNTech, which initially rolled out last fall. These vaccines target the ancestral COVID-19 strain and the omicron BA.4/5 subvariants.