RSV vaccine to protect infants gets green light from FDA advisers
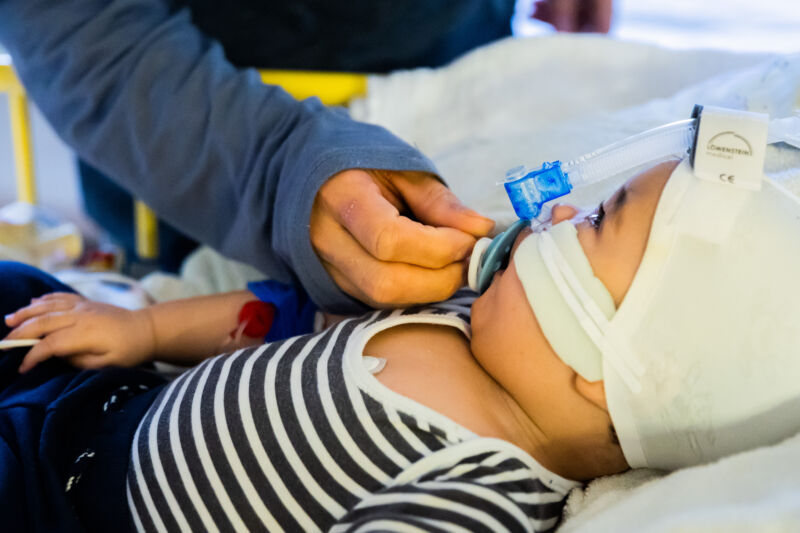
Enlarge / A father cares for his 8-and-a-half-month-old son, who is in the intensive care unit of the pediatric clinic at St. Joseph Hospital in Berlin with a respiratory infection and is receiving non-invasive ventilation (CPAP ventilation). (credit: Getty | Christoph Soeder)
A committee of independent expert advisers for the Food and Drug Administration on Thursday voted largely in favor of the agency approving Pfizer's vaccine for protecting infants from the common respiratory virus RSV-respiratory syncytial (sin-SISH-uhl) virus-which can be deadly to infants.
The vaccine, provisionally dubbed Abrysvo, is given to pregnant people between 24 and 36 weeks of pregnancy, allowing protective antibodies to develop and then cross the placenta to protect the fetus.
In a phase III trial involving nearly 7,400 pregnant people in 18 countries, the vaccine was nearly 82 percent effective at preventing severe RSV disease in the first 90 days of a baby's life. It was 69 percent effective at 180 days. In terms of protecting against non-severe respiratory disease from RSV, the trial results didn't meet the statistical criteria to find efficacy at 90 days, but data from 180 days suggested an efficacy of around 51 percent.