Process for producing ammonia that generates electricity instead of consuming energy. 500 million tons of ammonia are made each year for fertilizer
by noreply@blogger.com (brian wang) from NextBigFuture.com on (#2BFWD)
Nearly a century ago, German chemist Fritz Haber won the Nobel Prize in Chemistry for a process to generate ammonia from hydrogen and nitrogen gases. The process, still in use today, ushered in a revolution in agriculture, but now consumes around one percent of the world's energy to achieve the high pressures and temperatures that drive the chemical reactions to produce ammonia.
Today, University of Utah chemists publish a different method, using enzymes derived from nature, that generates ammonia at room temperature. As a bonus, the reaction generates a small electrical current.
Although chemistry and materials science and engineering professor Shelley Minteer and postdoctoral scholar Ross Milton have only been able to produce small quantities of ammonia so far, their method could lead to a less energy-intensive source of the ammonia, used worldwide as a vital fertilizer.
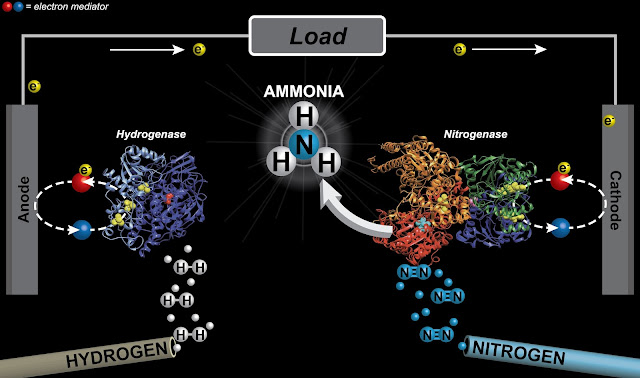

Angewandte Chemie International Edition- Bioelectrochemical Haber-Bosch Process: An Ammonia-Producing H2/N2 Fuel Cell
Read more










Today, University of Utah chemists publish a different method, using enzymes derived from nature, that generates ammonia at room temperature. As a bonus, the reaction generates a small electrical current.
Although chemistry and materials science and engineering professor Shelley Minteer and postdoctoral scholar Ross Milton have only been able to produce small quantities of ammonia so far, their method could lead to a less energy-intensive source of the ammonia, used worldwide as a vital fertilizer.


Angewandte Chemie International Edition- Bioelectrochemical Haber-Bosch Process: An Ammonia-Producing H2/N2 Fuel Cell
Read more