“Not uncommon at all”: AstraZeneca pauses COVID-19 vaccine trial
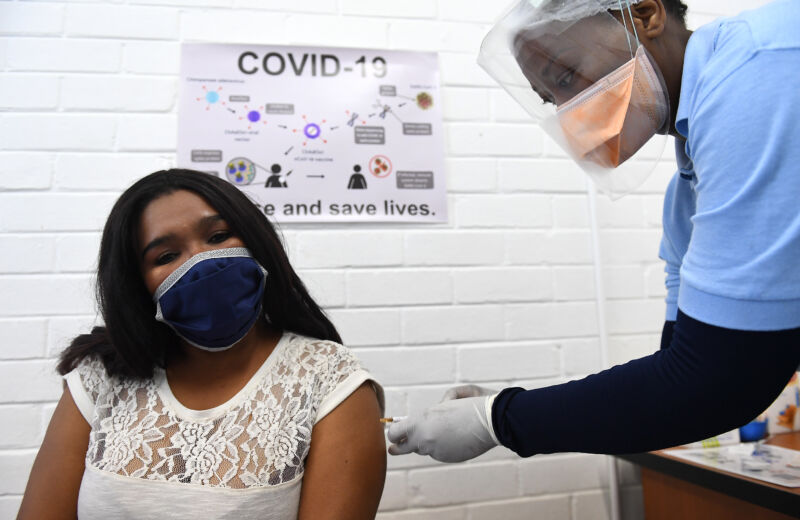
Enlarge / A volunteer receives an injection of AZD1222 from a medical worker during the country's first human clinical trial for a potential vaccine against COVID-19 at the Baragwanath Hospital on June 28, 2020, in Soweto, South Africa. It is reported that Africa's first COVID-19 vaccine trial began on June 24 in South Africa. (credit: Getty Images | Felix Dlangamandla)
With the coronavirus crisis gripping the globe, all eyes are on every bump and dip on the path to the pandemic's end. So, of course, news that researchers triggered a common pause to the clinical trials of a leading COVID-19 vaccine candidate made swift and alarming headlines late Tuesday.
The global phase III trials for the vaccine AZD1222 (formerly ChAdOx1)-developed by the University of Oxford and pharmaceutical giant AstraZeneca-were put on a temporary" and voluntary" pause for a standard review process," AstraZeneca said in a statement Wednesday.
According the company, the pause was triggered by a potentially unexplained illness" in one of thousands of participants involved in its trials. Per standard protocol, researchers must pause the trial to investigate whether the illness is related to exposure to the experimental vaccine or not.
Read 15 remaining paragraphs | Comments