Kids 5 to 11 get FDA OK for COVID-19 booster doses
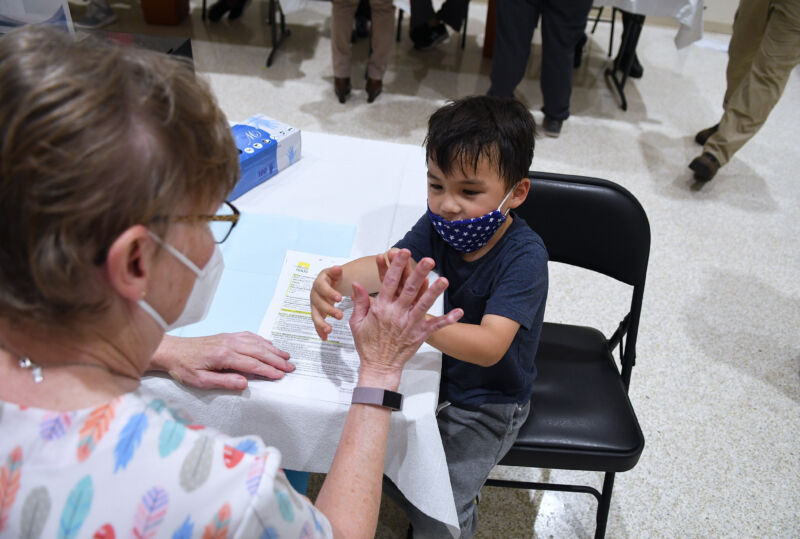
Enlarge / A boy gives a nurse a high five before receiving a shot of the Pfizer COVID-19 vaccine at a vaccination site for 5-11 year-olds at Eastmonte Park in Altamonte Springs, Florida. (credit: Getty | SOPA)
The Food and Drug Administration on Tuesday authorized booster doses of the Pfizer-BioNTech COVID-19 vaccine for children ages 5 through 11, the first booster dose for the age group intended to revive waning immune protection.
The authorization comes as the US continues to see COVID-19 cases rise due to the extremely transmissible omicron coronavirus subvariants, specifically BA.2 and BA.2.12.1, which now account for an estimated 51 percent and 47.5 percent of all reported cases, respectively. Transmission levels are considered high in just over 50 percent of US counties, according to the latest data from the Centers for Disease Control and Prevention. The seven-day average of new daily cases is nearly 96,000, up 57 percent in the last two weeks, according to data tracking by The New York Times. Hospitalizations are around 22,000, up 26 percent. Daily deaths are averaging around 300.
But some experts highlight that data on the current omicron-subvariant wave is muted because testing sites have shuttered, and many people are relying on at-home testing results that are largely not reported. Peter Hotez, a vaccine expert at Baylor College of Medicine, tweeted over the weekend that the current wave could rival that of the original omicron wave in January. He strongly urged Americans to get vaccinated and boosted and to vaccinate their children.
Read 6 remaining paragraphs | Comments